What is the relationship between the odor and structure of UV monomers?
Acrylate is widely used in the manufacture of various polymer materials, mainly because of its low temperature flexibility, heat resistance, aging resistance, high transparency and color stability. These properties allow it to be used in a very wide range of applications including plastics, floor varnishes, coatings, textiles, paints and adhesives. The type and amount of acrylate monomer used has a significant impact on the properties of the final product, including glass transition temperature, viscosity, hardness and durability. Copolymerization with monomers with hydroxyl, methyl or carboxyl functional groups can result in more polymers suitable for different applications.
The materials obtained by the polymerization of acrylate monomers are widely used in industry, but residual monomers are often found in polymerized materials. These residual monomers may not only cause problems such as skin irritation, but also cause an unpleasant odor in the final product due to the unpleasant odor of these monomers themselves.
The human olfactory system can perceive very low concentrations of acrylate monomers. For many acrylate polymer materials, most of the product odor comes from acrylate monomers. Different monomers have different odors, but what is the relationship between monomer structure and odor?
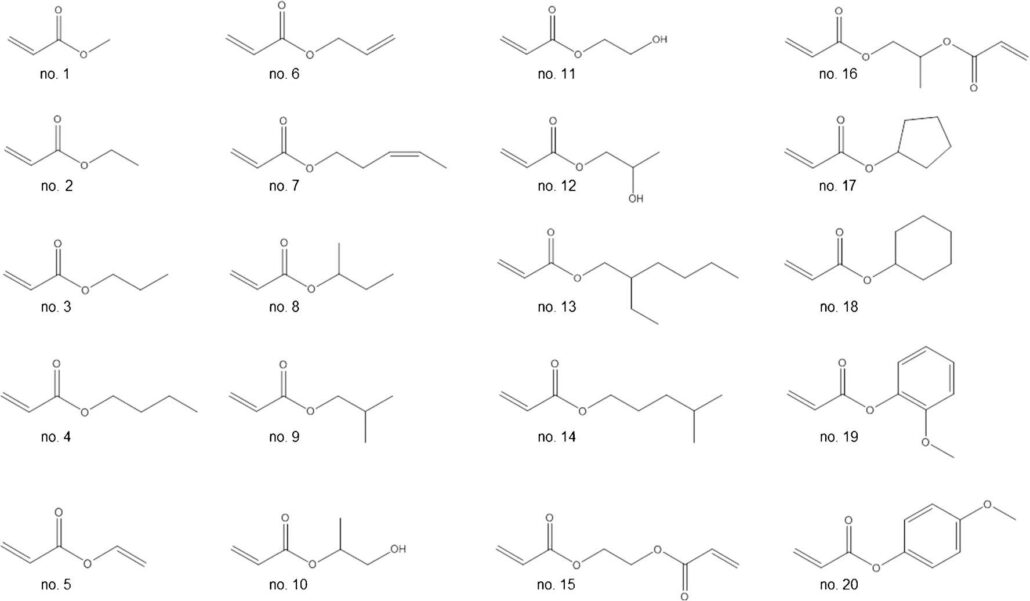
A total of 20 individual odors were tested for the study. These monomers include commercial and laboratory-synthesized ones. Tests have shown that the odors of these monomers can be classified as having sulfur, lighter gas, geranium and mushroom odors.
1,2-Propanediol diacrylate (no. 16), methyl acrylate (no. 1), ethyl acrylate (no. 2) and propyl acrylate (no. 3) are mainly described as sulfur and garlic odors. In addition, the latter two substances were also described as having a lighter gas odor, and ethyl acrylate and 1,2-propylene glycol diacrylate had the impression of a little glue odor. Vinyl acrylate (no. 5) and propylene acrylate (no. 6) were described as gaseous fuel odors, 1-hydroxyisopropyl acrylate (no. 10) and 2-hydroxyn-propyl acrylate (no. 12) Described as the smell of geranium and lighter gas. n-butyl acrylate (no. 4), 3-(Z)pentenyl acrylate (no. 7), sec-butyl acrylate (geranium, mushroom flavor; no. 8), 2-hydroxyethyl acrylate (no. 11), 4-methylpentyl acrylate (mushroom, fruity; no. 14) and ethylene glycol diacrylate (no. 15) were described as mushroom odors. Isobutyl acrylate (no. 9), 2-ethylhexyl acrylate (no. 13), cyclopentyl acrylate (no. 17) and cyclohexane acrylate (no. 18) are described as carrots Smell and geranium scent. 2-Methoxyphenyl acrylate (no. 19) smells like geranium and prosciutto, while its isomer 4-methoxyphenyl acrylate (no. 20) is described as anise and anise odor.
The odor thresholds of the tested monomers showed large differences. The odor threshold here refers to the concentration of the substance that produces the smallest stimulus to human odor perception, also known as the olfactory threshold. The higher the odor threshold, the lower the odor. From the experimental results, it can be seen that the odor threshold is more affected by functional groups than by chain length. Among the 20 tested monomers, the lowest odor thresholds were 2-methoxyphenyl acrylate (no. 19) and sec-butyl acrylate (no. 8), with odor thresholds of 0.068ng/Lair and 0.068ng/Lair, respectively. 0.073ng/Lair. 2-Hydroxy-n-propyl acrylate (no. 12) and 2-hydroxyethyl acrylate (no. 11) exhibited the highest odor thresholds at 106 ng/Lair and 178 ng/Lair, respectively, for acrylic- 5 and 9 times more than 2-ethylhexyl ester (no. 13).
| Polythiol/Polymercaptan | ||
| DMES Monomer | Bis(2-mercaptoethyl) sulfide | 3570-55-6 |
| DMPT Monomer | THIOCURE DMPT | 131538-00-6 |
| PETMP Monomer | PENTAERYTHRITOL TETRA(3-MERCAPTOPROPIONATE) | 7575-23-7 |
| PM839 Monomer | Polyoxy(methyl-1,2-ethanediyl) | 72244-98-5 |
| Monofunctional Monomer | ||
| HEMA Monomer | 2-hydroxyethyl methacrylate | 868-77-9 |
| HPMA Monomer | 2-Hydroxypropyl methacrylate | 27813-02-1 |
| THFA Monomer | Tetrahydrofurfuryl acrylate | 2399-48-6 |
| HDCPA Monomer | Hydrogenated dicyclopentenyl acrylate | 79637-74-4 |
| DCPMA Monomer | Dihydrodicyclopentadienyl methacrylate | 30798-39-1 |
| DCPA Monomer | Dihydrodicyclopentadienyl Acrylate | 12542-30-2 |
| DCPEMA Monomer | Dicyclopentenyloxyethyl Methacrylate | 68586-19-6 |
| DCPEOA Monomer | Dicyclopentenyloxyethyl Acrylate | 65983-31-5 |
| NP-4EA Monomer | (4) ethoxylated nonylphenol | 50974-47-5 |
| LA Monomer | Lauryl acrylate / Dodecyl acrylate | 2156-97-0 |
| THFMA Monomer | Tetrahydrofurfuryl methacrylate | 2455-24-5 |
| PHEA Monomer | 2-PHENOXYETHYL ACRYLATE | 48145-04-6 |
| LMA Monomer | Lauryl methacrylate | 142-90-5 |
| IDA Monomer | Isodecyl acrylate | 1330-61-6 |
| IBOMA Monomer | Isobornyl methacrylate | 7534-94-3 |
| IBOA Monomer | Isobornyl acrylate | 5888-33-5 |
| EOEOEA Monomer | 2-(2-Ethoxyethoxy)ethyl acrylate | 7328-17-8 |
| Multifunctional monomer | ||
| DPHA Monomer | Dipentaerythritol hexaacrylate | 29570-58-9 |
| DI-TMPTA Monomer | DI(TRIMETHYLOLPROPANE) TETRAACRYLATE | 94108-97-1 |
| Acrylamide monomer | ||
| ACMO Monomer | 4-acryloylmorpholine | 5117-12-4 |
| Di-functional Monomer | ||
| PEGDMA Monomer | Poly(ethylene glycol) dimethacrylate | 25852-47-5 |
| TPGDA Monomer | Tripropylene glycol diacrylate | 42978-66-5 |
| TEGDMA Monomer | Triethylene glycol dimethacrylate | 109-16-0 |
| PO2-NPGDA Monomer | Propoxylate neopentylene glycol diacrylate | 84170-74-1 |
| PEGDA Monomer | Polyethylene Glycol Diacrylate | 26570-48-9 |
| PDDA Monomer | Phthalate diethylene glycol diacrylate | |
| NPGDA Monomer | Neopentyl glycol diacrylate | 2223-82-7 |
| HDDA Monomer | Hexamethylene Diacrylate | 13048-33-4 |
| EO4-BPADA Monomer | ETHOXYLATED (4) BISPHENOL A DIACRYLATE | 64401-02-1 |
| EO10-BPADA Monomer | ETHOXYLATED (10) BISPHENOL A DIACRYLATE | 64401-02-1 |
| EGDMA Monomer | Ethylene glycol dimethacrylate | 97-90-5 |
| DPGDA Monomer | Dipropylene Glycol Dienoate | 57472-68-1 |
| Bis-GMA Monomer | Bisphenol A Glycidyl Methacrylate | 1565-94-2 |
| Trifunctional Monomer | ||
| TMPTMA Monomer | Trimethylolpropane trimethacrylate | 3290-92-4 |
| TMPTA Monomer | Trimethylolpropane triacrylate | 15625-89-5 |
| PETA Monomer | Pentaerythritol triacrylate | 3524-68-3 |
| GPTA ( G3POTA ) Monomer | GLYCERYL PROPOXY TRIACRYLATE | 52408-84-1 |
| EO3-TMPTA Monomer | Ethoxylated trimethylolpropane triacrylate | 28961-43-5 |
| Photoresist Monomer | ||
| IPAMA Monomer | 2-isopropyl-2-adamantyl methacrylate | 297156-50-4 |
| ECPMA Monomer | 1-Ethylcyclopentyl Methacrylate | 266308-58-1 |
| ADAMA Monomer | 1-Adamantyl Methacrylate | 16887-36-8 |
| Methacrylates monomer | ||
| TBAEMA Monomer | 2-(Tert-butylamino)ethyl methacrylate | 3775-90-4 |
| NBMA Monomer | n-Butyl methacrylate | 97-88-1 |
| MEMA Monomer | 2-Methoxyethyl Methacrylate | 6976-93-8 |
| i-BMA Monomer | Isobutyl methacrylate | 97-86-9 |
| EHMA Monomer | 2-Ethylhexyl methacrylate | 688-84-6 |
| EGDMP Monomer | Ethylene glycol Bis(3-mercaptopropionate) | 22504-50-3 |
| EEMA Monomer | 2-ethoxyethyl 2-methylprop-2-enoate | 2370-63-0 |
| DMAEMA Monomer | N,M-Dimethylaminoethyl methacrylate | 2867-47-2 |
| DEAM Monomer | Diethylaminoethyl methacrylate | 105-16-8 |
| CHMA Monomer | Cyclohexyl methacrylate | 101-43-9 |
| BZMA Monomer | Benzyl methacrylate | 2495-37-6 |
| BDDMP Monomer | 1,4-Butanediol Di(3-mercaptopropionate) | 92140-97-1 |
| BDDMA Monomer | 1,4-Butanedioldimethacrylate | 2082-81-7 |
| AMA Monomer | Allyl methacrylate | 96-05-9 |
| AAEM Monomer | Acetylacetoxyethyl methacrylate | 21282-97-3 |
| Acrylates Monomer | ||
| IBA Monomer | Isobutyl acrylate | 106-63-8 |
| EMA Monomer | Ethyl methacrylate | 97-63-2 |
| DMAEA Monomer | Dimethylaminoethyl acrylate | 2439-35-2 |
| DEAEA Monomer | 2-(diethylamino)ethyl prop-2-enoate | 2426-54-2 |
| CHA Monomer | cyclohexyl prop-2-enoate | 3066-71-5 |
| BZA Monomer | benzyl prop-2-enoate | 2495-35-4 |
Contact Us Now!
