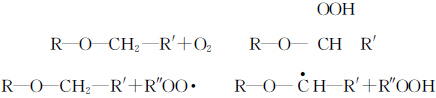紫外线单体对紫外线固化氧气阻隔有什么影响?
1.2 样品制备和测试
准确称量单体和引发剂(其中单体未经提纯),并用磁力搅拌器搅拌均匀。在静态空气气氛和氮气气氛下使用紫外-DSC 进行紫外固化实验,每次取样量相等,使样品在坩埚中的厚度相同。紫外-DSC 程序设定样品温度恒定 2 分钟,然后程序自动打开光源,紫外光(波长范围 325-400 nm)由玻璃纤维引入 DSC 池,照射样品坩埚和参比坩埚,DSC 测量样品光固化过程的热流值。测得的光强为 48.27 mW-cm-2。通过在样品坩埚底部涂抹炭黑来确定光强度,然后用紫外-DSC 测量光吸收的能量,再除以坩埚底部面积得出光强度。
2 结果与讨论
2.1 数据处理
光固化率的计算公式为
光固化率 R=dC/dt=(dH/dt)/Hmax,其中 dH/dt 是 UV-DSC 测得的光固化过程焓随时间变化曲线上的热流值;Hmax 是光照 200 秒时样品的总聚合热,由光固化放热峰积分得到。光固化过程中最大固化速率对应的时间为 tmax。自由基聚合会发生自动加速现象,光固化速率曲线以时间为导数,曲线最大值对应的时间记为 tamax,此时可认为是光固化速率增长最快的时间,此时体系中氧的阻隔和笼效应等因素对初级自由基的消耗达到最小。自由基引发单体开始快速聚合。
计算样品中的引发剂含量(摩尔分数)α:
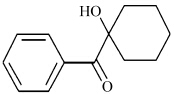
其中 n 引发剂为体系中引发剂的摩尔量,f 为单体的官能度,n 单体为体系中单体的摩尔量。图 1 显示的是裂解型引发剂 Irgacure-184 的分子结构,式(1)中的 "2 "表示理论上一分子引发剂在光照射下可以分解成两分子自由基。
Irgacure-184 的结构式
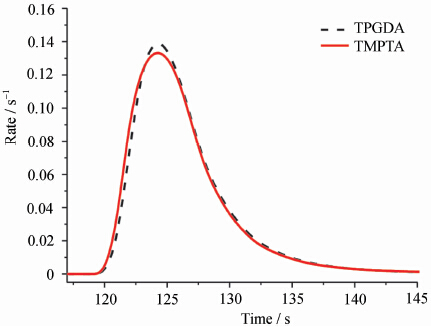
图 2 显示了在 4% 起始剂含量(质量分数)下不同单体光固化的速率曲线。从图中可以看出,对于不同单体,在固化前期的固化速率存在差异,这种差异也用时间表示,说明不同单体紫外光固化在时间上存在速度差异。将单体光固化速率增长最快时的时间 tamax 和最大光固化速率对应的时间 tmax 与引发剂含量的变化曲线进行比较,结果如图 3 所示。
不同单体与 4% 引发剂的紫外线固化速率曲线
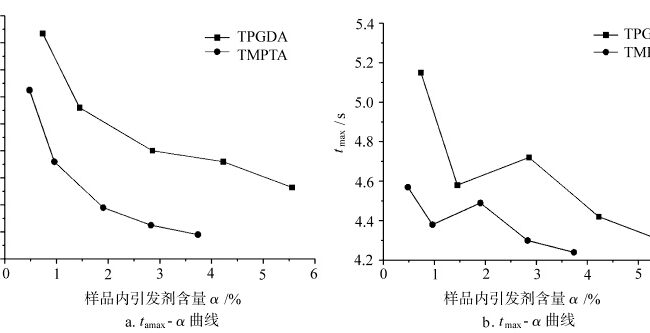
的关系 t最大值 和 t最大 不同单体在空气中固化过程中引发剂含量的变化
图 3 显示了不同单体在空气中光固化的 tmax 和 tamax 曲线与样品中引发剂含量 α 的函数关系。在固化初期,当氧气存在时,光引发剂在光照下产生的活性自由基一部分被溶解在样品中的氧气消耗掉,另一部分引发单体聚合。氧阻断效应消耗了引发剂分解产生的自由基和单体自由基,从而影响光固化的 tamax 和 tmax 的大小,进而反映出光固化对氧气和引发剂含量的敏感性。从图 3a 中可以看出,每种单体光固化的 tamax 都会随着引发剂含量的增加而按一定规律减小。在图 3b 中,随着引发剂含量的增加,各单体光固化的 tmax 出现了一定的波动。这是因为当固化反应速率达到最大值时,体系已达到较高的固化度,分子链段因玻璃化效应而逐渐冻结,此时光固化反应的 tmax 对引发剂含量的敏感性降低。而 tamax 在光固化初期,当体系固化程度不高时,光固化反应对体系中引发剂的含量更为敏感。因此,相比之下,塔迈斯更能反映光固化对引发剂含量的敏感性。
2.2 大气对不同单体光固化塔玛斯的影响
在空气环境中,由于氧气的阻隔作用,光固化材料的 tamax 值与氮气环境中的应有所不同。在空气和氮气环境下,当加入较少的引发剂时,对单体 TMPTA 进行了 UV-DSC 测试,结果见表 1。
| TMPTA 的 tamaxtamax 紫外线固化,在空气或 N2 中的引发剂含量较低 | ||
| 样品中引发剂的含量/% | 三羟甲基氨基甲烷 | |
| 气 | N2 | |
| 0.48 | 2.65 | 2.18 |
| 0.96 | 2.12 | 1.92 |
t最大值 TMPTA 在空气或氮气中用紫外线固化,引发剂含量低2
表 1 显示了在空气和氮气中分别添加较低水平的引发剂光固化 TMPTA 的 tamax 值比较。从上表可以看出,当引发剂含量相同时,在空气下光固化的 tamax 要大于在氮气下的 tamax,这表明在空气下光固化会受到氧气阻隔的影响。一般认为,溶解在原料中和表面的氧气在光固化过程中会发生以下反应。
生成的过氧化物自由基 ROO- 非常稳定,无法引发聚合反应。氧气不仅能清除光固化过程中引发剂产生的自由基,还能终止单体自由基。
不同单体的光固化对阻氧聚合的敏感性不同,与单体官能度、双键活性和结构等因素有关 [13]。为了研究单体结构对阻氧聚合的影响,我们选择了两种不同的环氧乙烷化 TMPTA,分别在空气和氮气条件下进行 UV-DSC 实验。除了环氧乙烷基团的数量外,这些同源单体都具有相似的结构,这就避免了因选择其他一些结构差异较大的单体而使问题复杂化[13]。
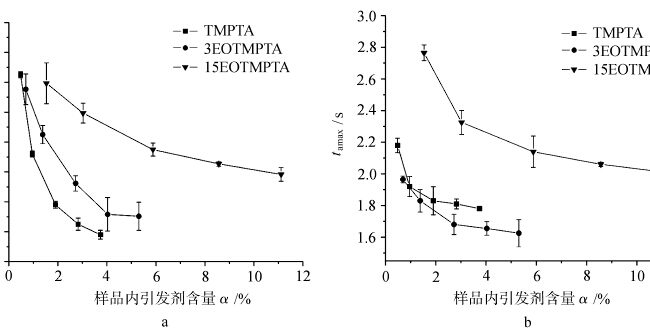
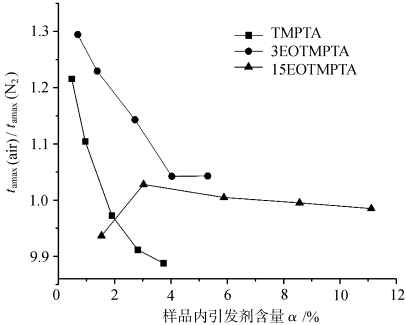
图 4 显示了在空气和氮气条件下光固化具有不同引发剂的 TMPTA 单体时,样品中的 tamax 随引发剂含量的变化情况。从图中可以看出,在空气和氮气条件下光固化的每种单体的 tamax 都随着引发剂含量的增加而减少。每种单体在空气和氮气条件下光固化的 tamax 随单体中 EO 含量的变化而变化。这说明单体中环氧乙烷的含量对光固化塔马克思有影响。对于同一种单体,当其他条件相同时,在空气和氮气条件下光固化塔麻的差异与氧气阻隔性有关。
tamax(air)/tamax(N2)表示单体在空气和氮气条件下光固化的tamax之差,从而反映了各单体光固化对氧气阻隔作用的大小,其值越大,氧气阻隔作用越强。从图 5 中可以看出,单体 TMPTA 和单体 3EOTMPTA 的 tamax(air)/tamax(N2)比值在引发剂含量较小时较大,随着引发剂含量的增加而减小。这表明,当引发剂含量较少时,单体 TMPTA 和单体 3EOTMPTA 受氧阻断效应的影响更明显。当单体中的环氧乙烷含量达到 15 时,单体的 tamax(空气)/tamax(N2)比值接近 1,也就是说、EO 分子中的类醚结构 -O-CH2- 上的α-H 很容易被氧取代,并发生以下反应。
的关系 t最大值(空气)/t最大值(N2) 与不同乙氧基化 TMPTA 固化过程中引发剂含量的关系。
α-H 一方面可以消耗体系中的部分氧气,另一方面可以终止由自由基和氧气生成的过氧自由基 ROO-,从而减少活性自由基的消耗,并将引发活性重新导向具有引发活性的链段,从而减少光固化过程中的氧阻塞。
2.3 单体中 α-H 的含量对光固化氧阻聚的影响
为了研究氧阻断与单体 EO 基团中 α-H 数量之间的关系,将图 4 中的 tamax-α 曲线与不同方程进行线性拟合,发现线性拟合方程 lnt=a+b/α0.5 得到的相关系数最高。表 2 列出了拟合得到的斜率 b 和相关系数 R。
| 通过线性拟合得出 b 值和 R 值 1/α0.5 和 lnt | ||||
| 单体 | 气 | N2 | ||
| b | R | b | R | |
| TMPTA | 0.056 | 0.99894 | 0.0213 | 0.95494 |
| EO3TMPTA | 0.0554 | 0.95877 | 0.0257 | 0.98564 |
| 15EOTMPTA | 0.0531 | 0.96578 | 0.062 | 0.98944 |
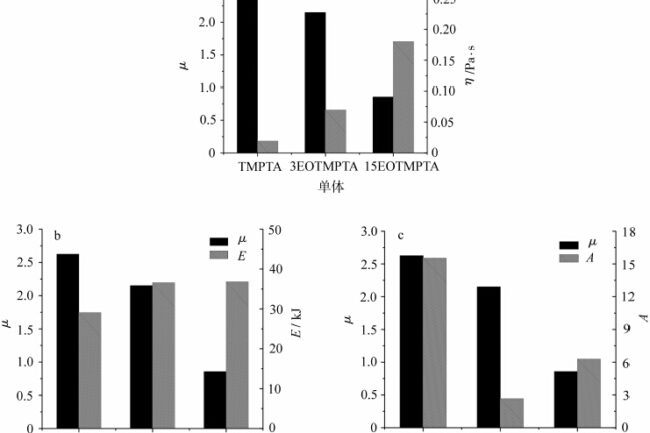
比较图 6 中不同单体对应的 μ 值,当单体为 TMPTA 时,μ 值最大,即单体 TMPTA 在紫外固化时的阻氧效果最好。随着单体中 EO 含量的增加,单体中 α-H 的含量也随之增加,μ 值随之减小,这表明氧阻隔性与单体中 α-H 的含量有关,α-H 能捕获体系中的氧和过氧自由基,减轻氧阻隔性。粘度是一种与分子结构相关的性质,涉及单体的流动性能,因此有必要进一步探讨单体流动性能对氧障聚合的影响。
2.4 单体的流动特性对氧障聚集的影响
系统的流动特性与分子结构有关。一般来说,分子链越柔韧,链内的旋转障碍越小,作为移动单元的链段越短,柔韧分子链的活化能越低,流动性越好。分子量越大,分子运动的内摩擦阻力越大,流动性越差[19]。因此,在 TMPTA 单体中引入环氧乙烷基团会改变体系的流动特性。使用恒定剪切速率为 19.1 /min 和温度上升速率为 2 °C /min 的流变仪,测量了每种单体的粘度随温度变化的曲线。表 3 列出了计算得出的流动活化能 E、前置因子 A 和 25 ℃ 时的单体粘度 η。
| 单体的粘度 η(25 ℃)、流动活化能 E 和前指数因子 A 的值 | |||
| 单体 | η/Pa-s(25℃) | A/10-8 | E/J |
| TMPTA | 0.01934 | 15.53 | 29079.88 |
| 3EOTMPTA | 0.06948 | 2.65 | 36600.47 |
| 15EOTMPTA | 0.18016 | 6.28 | 36821.87 |
其中,流动活化能 E 与分子结构有关,受分子链的刚度和柔度、侧基的大小和极性等因素的影响。指数前沿因子 A 是一个与分子量有关的常数。为了研究单体的流动特性与氧阻隔性之间的关系,将不同单体的 μ 值与相应的粘度 η、流动活化能 E 和前置因子 A 相关联,如图 7 所示。
图 7 表示不同单体的 μ 值分别与流动活化能 E、指锋因子 A 和粘度 η 的关系比较。从图 7a 中可以看出,随着单体中 EO 数量的增加和单体粘度的增大,μ 值呈下降趋势。氧阻聚的大小与氧的浓度密切相关,氧的浓度越大,氧阻聚就越明显[7]。在光固化体系内部,溶解氧的量是一定的,光固化过程中氧对自由基的淬灭和清除速率极快,消耗的氧主要靠空气中的氧不断向体系内部扩散来补充,这必然涉及到体系中氧的运动能力,而体系中氧的运动能力与体系的粘度有关。当体系的粘度较低时,空气中的氧气更容易扩散到体系内部,表现出较大的氧气阻滞作用,因此单体 TMPTA 的 μ 值较高,对氧气的阻滞作用明显。当体系的粘度增大时,体系中氧的移动能力下降,来不及补充清除自由基消耗的氧,氧的阻滞作用减弱。从图中可以看出,当单体中的环氧乙烷含量增加到 15 时,单体粘度继续增加,μ 值趋于 1,即空气和氮气下的光固化差异不大。
然后将单体的流动活化能和指前因子与 μ 值进行比较。从图 7b 中可以看出,随着单体中 EO 基团的引入,单体的流动活化能从 TMPTA 增加到 3EOTMPTA。当单体中的环氧乙烷数量从 3 个增加到 15 个时,流动活化能基本保持不变,这表明单体中环氧乙烷数量的增加对流动活化能基本没有影响。这是因为虽然单体中的环氧乙烷数量增加了,但运动单位的数量并没有发生变化,这说明对于环氧乙烷化的 TMPTA 单体来说,流动活化能对氧障的影响并不显著。从图 7c 中可以看出,从 TMPTA 到 3EOTMPTA,指前因子 A 发生了很大变化,这可能与 EO 基团的存在或不存在有关。随着单体中 EO 数量的增加,前因子 A 也随之增加,这是由于单体中 EO 数量的增加导致单体分子量的增加。可以看出,对于环氧乙烷化 TMPTA 单体,分子中环氧乙烷数量的增加会导致分子量的增加,从而导致单体粘度的增加,进而降低氧气阻隔性。对于聚合材料来说,一般可以通过增加分子链的刚性、增加侧基的极性、增加分子量等方法来增加体系的粘度。可以推测,通过适当的配方组成,如引入含有活性氢的结构、增加刚性分子的比例、增加分子量等,可以减少光固化氧阻隔的不利影响。
3 结论
(1) 增加单体分子中 EO 基团的数量可减少光固化过程中的氧阻聚。这与该基团中的α-H 能捕获氧和过氧自由基以及体系粘度增加有关。
(2) 对于本研究选择的几种环氧乙烷化 TMPTA,单体的流动活化能对氧气阻隔聚合的影响很小。单体中环氧乙烷含量的增加会导致其分子量的增加,从而导致单体粘度的增加,这反过来又会对阻氧聚集产生影响。
紫外线单体 同系列产品
| 聚硫醇/聚硫醇 | ||
| DMES 单体 | 双(2-巯基乙基)硫醚 | 3570-55-6 |
| DMPT 单体 | THIOCURE DMPT | 131538-00-6 |
| PETMP 单体 | 7575-23-7 | |
| PM839 单体 | 聚氧(甲基-1,2-乙二基) | 72244-98-5 |
| 单官能团单体 | ||
| HEMA 单体 | 甲基丙烯酸 2-羟乙基酯 | 868-77-9 |
| HPMA 单体 | 甲基丙烯酸羟丙酯 | 27813-02-1 |
| THFA 单体 | 丙烯酸四氢糠酯 | 2399-48-6 |
| HDCPA 单体 | 氢化双环戊烯丙烯酸酯 | 79637-74-4 |
| DCPMA 单体 | 甲基丙烯酸二氢双环戊二烯酯 | 30798-39-1 |
| DCPA 单体 | 丙烯酸二氢双环戊二烯酯 | 12542-30-2 |
| 二氯丙烯酰亚胺单体 | 甲基丙烯酸二环戊氧基乙酯 | 68586-19-6 |
| DCPEOA 单体 | 丙烯酸二环戊烯基氧基乙基酯 | 65983-31-5 |
| NP-4EA 单体 | (4) 乙氧基化壬基酚 | 50974-47-5 |
| LA 单体 | 丙烯酸十二烷基酯/丙烯酸十二烷基酯 | 2156-97-0 |
| THFMA 单体 | 甲基丙烯酸四氢糠酯 | 2455-24-5 |
| PHEA 单体 | 2-苯氧基乙基丙烯酸酯 | 48145-04-6 |
| LMA 单体 | 甲基丙烯酸月桂酯 | 142-90-5 |
| IDA 单体 | 丙烯酸异癸酯 | 1330-61-6 |
| IBOMA 单体 | 甲基丙烯酸异冰片酯 | 7534-94-3 |
| IBOA 单体 | 丙烯酸异冰片酯 | 5888-33-5 |
| EOEOEA 单体 | 2-(2-乙氧基乙氧基)丙烯酸乙酯 | 7328-17-8 |
| 多功能单体 | ||
| DPHA 单体 | 29570-58-9 | |
| DI-TMPTA 单体 | 二(三羟甲基丙烷)四丙烯酸酯 | 94108-97-1 |
| 丙烯酰胺单体 | ||
| ACMO 单体 | 4-丙烯酰基吗啉 | 5117-12-4 |
| 双功能单体 | ||
| PEGDMA 单体 | 聚乙二醇二甲基丙烯酸酯 | 25852-47-5 |
| TPGDA 单体 | 三丙二醇二丙烯酸酯 | 42978-66-5 |
| TEGDMA 单体 | 三乙二醇二甲基丙烯酸酯 | 109-16-0 |
| PO2-NPGDA 单体 | 丙氧基新戊二醇二丙烯酸酯 | 84170-74-1 |
| PEGDA 单体 | 聚乙二醇二丙烯酸酯 | 26570-48-9 |
| PDDA 单体 | 邻苯二甲酸二乙二醇二丙烯酸酯 | |
| NPGDA 单体 | 新戊二醇二丙烯酸酯 | 2223-82-7 |
| HDDA 单体 | 二丙烯酸六亚甲基酯 | 13048-33-4 |
| EO4-BPADA 单体 | 乙氧基化 (4) 双酚 A 二丙烯酸酯 | 64401-02-1 |
| EO10-BPADA 单体 | 乙氧基化 (10) 双酚 A 二丙烯酸酯 | 64401-02-1 |
| EGDMA 单体 | 乙二醇二甲基丙烯酸酯 | 97-90-5 |
| DPGDA 单体 | 二丙二醇二烯酸酯 | 57472-68-1 |
| 双-GMA 单体 | 双酚 A 甲基丙烯酸缩水甘油酯 | 1565-94-2 |
| 三官能单体 | ||
| TMPTMA 单体 | 三羟甲基丙烷三甲基丙烯酸酯 | 3290-92-4 |
| TMPTA 单体 | 三羟甲基丙烷三丙烯酸酯 | 15625-89-5 |
| PETA 单体 | 3524-68-3 | |
| GPTA ( G3POTA ) 单体 | 丙氧基三丙烯酸甘油酯 | 52408-84-1 |
| EO3-TMPTA 单体 | 三羟甲基丙烷三丙烯酸乙氧基化物 | 28961-43-5 |
| 光阻单体 | ||
| IPAMA 单体 | 2-异丙基-2-金刚烷基甲基丙烯酸酯 | 297156-50-4 |
| ECPMA 单体 | 1-乙基环戊基甲基丙烯酸酯 | 266308-58-1 |
| ADAMA 单体 | 1-金刚烷基甲基丙烯酸酯 | 16887-36-8 |
| 甲基丙烯酸酯单体 | ||
| TBAEMA 单体 | 2-(叔丁基氨基)乙基甲基丙烯酸酯 | 3775-90-4 |
| NBMA 单体 | 甲基丙烯酸正丁酯 | 97-88-1 |
| MEMA 单体 | 甲基丙烯酸 2-甲氧基乙酯 | 6976-93-8 |
| i-BMA 单体 | 甲基丙烯酸异丁酯 | 97-86-9 |
| EHMA 单体 | 甲基丙烯酸 2-乙基己酯 | 688-84-6 |
| EGDMP 单体 | 乙二醇双(3-巯基丙酸酯) | 22504-50-3 |
| EEMA 单体 | 2-甲基丙-2-烯酸 2-乙氧基乙酯 | 2370-63-0 |
| DMAEMA 单体 | 甲基丙烯酸 N,M-二甲基氨基乙酯 | 2867-47-2 |
| DEAM 单体 | 甲基丙烯酸二乙氨基乙酯 | 105-16-8 |
| CHMA 单体 | 甲基丙烯酸环己基酯 | 101-43-9 |
| BZMA 单体 | 甲基丙烯酸苄酯 | 2495-37-6 |
| BDDMP 单体 | 1,4-丁二醇二(3-巯基丙酸酯) | 92140-97-1 |
| BDDMA 单体 | 1,4-丁二醇二甲基丙烯酸酯 | 2082-81-7 |
| AMA 单体 | 甲基丙烯酸烯丙酯 | 96-05-9 |
| AAEM 单体 | 甲基丙烯酸乙酰乙酰氧基乙基酯 | 21282-97-3 |
| 丙烯酸酯单体 | ||
| IBA 单体 | 丙烯酸异丁酯 | 106-63-8 |
| EMA 单体 | 甲基丙烯酸乙酯 | 97-63-2 |
| DMAEA 单体 | 丙烯酸二甲胺基乙酯 | 2439-35-2 |
| DEAEA 单体 | 2-(二乙基氨基)乙基丙-2-烯酸酯 | 2426-54-2 |
| CHA 单体 | 丙-2-烯酸环己基酯 | 3066-71-5 |
| BZA 单体 | 丙-2-烯酸苄酯 | 2495-35-4 |