Overview of enzyme immobilization methods
In the previous article, we mentioned that the bitter substances in citrus fruits are mainly naringin, which can be gradually decomposed into glucose and naringenin by naringinase, and finally achieve the purpose of debittering. In the actual production and use process, compared with free enzymes, immobilized enzymes not only have higher catalytic efficiency and better specificity, but also enhance resistance to harsh conditions and prolong the service life of enzymes; at the same time, immobilized enzymes is convenient to separate from the reaction product, simplify the production process, and reduce the cost. Therefore, here is a brief introduction to the immobilization methods of common enzymes.
Immobilized enzyme refers to an enzyme that is fixed on a certain carrier to form water insolubility and still has catalytic properties. As early as 1954, Glubhofer and Schleith used the diazo method to modify polystyrene resin for the immobilization of pepsin and carboxypeptidase to prepare immobilized enzymes. Then in 1969, Japanese scientist Chi Yanichiro used immobilized acylated amino acid to realize continuous production of L-amino acid for the first time, which promoted the rapid development of immobilization technology in enzyme engineering. When immobilized enzymes appeared, there were many different names, but at the first International Enzyme Engineering Conference in 1971, it was formally proposed to use “immobilized enzymes” as a proper term. The enzyme-catalyzed reaction has mild conditions, high catalytic efficiency, and highly specificity. At the same time, the enzyme is easy to degrade and is environmentally friendly. The immobilized enzyme not only retains these characteristics of the enzyme, but also has many properties that are difficult for free enzymes to possess, such as: ① easy to separate from the reaction system; ② recyclable for recycling; ③ improving the stability of the enzyme under harsh conditions; ④ It can realize enzyme catalysis in non-aqueous phase; ⑤ It can produce a multi-enzyme system with continuous reaction. Because immobilized enzymes can have important application value in the fields of medicine, food, environmental protection, etc., they have attracted the attention of scholars in recent years and have many applications in industry.

Common enzyme immobilization methods mainly include adsorption method, embedding method, cross-linking method and covalent coupling method. The first two belong to the physical method, and the latter two belong to the chemical method. The principle of those methods to immobilize enzyme is shown in Figure 1.
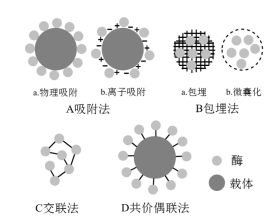
Figure 1 Method of immobilizing enzyme
1 Adsorption method
The adsorption method is a method to complete the immobilization through the surface adsorption of the carrier to the enzyme or through the positive and negative charge interaction between the carrier and the enzyme, including physical adsorption and ion adsorption.

-
physical adsorption
The physical adsorption method is a method of fixing the enzyme on the surface of the carrier using the adsorption effect of the carrier on the enzyme. The method is simple to operate, does not require modification and activation, and the active center of the enzyme is well preserved, but the binding force is low, and its force is mainly intermolecular force and surface tension. The adsorbents commonly used for enzyme immobilization include activated carbon, graphene, diatomaceous earth and other non-water-soluble adsorbents with strong water absorption capacity. Zhao et al. used reduced graphene oxide as a carrier to co-immobilize glucose oxidase and glucoamylase to prepare an immobilized composite enzyme.

-
ion adsorption
The ion adsorption method is a method of immobilizing the enzyme through the positive and negative charge interaction between the carrier and the enzyme. The method has mild preparation conditions, and the enzyme activity is not easy to lose, but the binding force is also weak and easy to fall off. Mateo et al. prepared a new type of ion exchange resin to fix lipase, β-galactosidase and amino acid oxidase respectively. Under the conditions of pH 7.0 and 4 ℃, the enzyme can reach adsorption saturation within a few minutes, and the enzyme activity recovery rate up to 100%, showing a good immobilization effect.

2 Embedding method
The embedding method is a method in which the enzyme is embedded in a porous material, and includes gel embedding method and semi-permeable membrane embedding method. The gel embedding method is a fixation method in which the enzyme is embedded in the internal network structure of the gel (Figure 1B-a). Zhang Shuxiang and others used sodium alginate to embed fungal laccase to treat low-concentration papermaking wastewater. The maximum recovery rate of enzyme activity was 48%, and the enzyme activity remained 64% after 8 runs. The semi-permeable membrane embedding method is a method of immobilizing enzymes in a semi-permeable membrane made of high molecular polymers (Figure 1B-b). This method also does not require chemical modification and does not affect the activity of the enzyme, but it is not easy to control the size of the membrane. It is difficult to maintain enzyme activity while controlling the free entry and exit of reactants and products without enzyme leakage. Therefore, this method is usually combined with other methods in practical use. Rilling P et al. used a carrier to immobilize protein molecules together in a microcapsule, which not only increased the stability of the immobilized enzyme, but also improved its properties.

3 Cross-linking method
The cross-linking method is a method in which the enzyme molecules are cross-linked with each other using bifunctional or multifunctional reagents, and the enzyme molecules and the reagents form a covalent bond and are fixed. The immobilized enzyme prepared by this method has good stability, but some groups of the enzyme such as amino, sulfhydryl, imidazolyl, etc., may participate in the cross-linking reaction. If the selected cross-linking agent and functional group are not appropriate, the structure of the active center of the enzyme may be destroyed, resulting in a serious loss of enzyme activity, so this method is usually combined with other methods. Li Xiaojing and others first encapsulated pepsin with sodium alginate solution, then added dropwise to a solution containing a certain concentration of chitosan and CaCl2 to obtain immobilized enzyme microspheres, then added a certain amount of glutaraldehyde for cross-linking to obtain immobilized pepsin. This method obtained a immobilized enzyme with both good operational stability and thermal stability.

4 Covalent coupling method
The covalent coupling method (also known as the covalent binding method) is a method of immobilizing the enzyme by forming a covalent bond between the enzyme and the carrier (as shown in Figure 2).
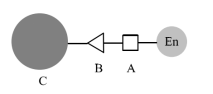
Figure 2 Immobilized enzyme by covalent binding method, A-active amino acid residue; B-binding group on carrier; C-carrier.

Covalent binding methods generally include three types: (1) the carrier is modified and activated, and then covalently bound to the enzyme molecule; (2) the carrier and the enzyme molecule are covalently bound through a coupling agent; (3) the enzyme molecule is modified after activation, it is combined with the carrier. Among them, the first type is more commonly used. After activating the carrier to connect certain active groups such as amino, epoxy, etc., to the active residue of the enzyme molecule (such as amino, carboxyl, hydroxyl, etc.), the enzyme is immobilized. This method is much more reliable than the adsorption method. At the same time, because the carrier is usually larger, the amino acid residues involved in the reaction on the enzyme are generally those exposed on the periphery of the enzyme, and the effect on the active center of the enzyme is relatively small. This is an excellent method of immobilizing enzymes. Yan Keliang and others used aminosilanized magnetic nanoparticles to immobilize pectinase, and the immobilization rate and enzyme activity recovery rate were 44.44% and 40.86%, respectively, achieving a good immobilization effect. The next article will continue to introduce in detail the different methods of preparing magnetic biopolymer microspheres.

Contact Us Now!
If you need Price, please fill in your contact information in the form below, we will usually contact you within 24 hours. You could also email me info@longchangchemical.com during working hours ( 8:30 am to 6:00 pm UTC+8 Mon.~Sat. ) or use the website live chat to get prompt reply.
| Compound Glucoamylase | 9032-08-0 |
| Pullulanase | 9075-68-7 |
| Xylanase | 37278-89-0 |
| Cellulase | 9012-54-8 |
| Naringinase | 9068-31-9 |
| β-Amylase | 9000-91-3 |
| Glucose oxidase | 9001-37-0 |
| alpha-Amylase | 9000-90-2 |
| Pectinase | 9032-75-1 |
| Peroxidase | 9003-99-0 |
| Lipase | 9001-62-1 |
| Catalase | 9001-05-2 |
| TANNASE | 9025-71-2 |
| Elastase | 39445-21-1 |
| Urease | 9002-13-5 |
| DEXTRANASE | 9025-70-1 |
| L-Lactic dehydrogenase | 9001-60-9 |
| Dehydrogenase malate | 9001-64-3 |
| Cholesterol oxidase | 9028-76-6 |
