Why use a composite photoinitiator in light curing formulating?
Photoinitiators are a very important component of photocurable formulations and are a source of free radicals. However, excessive use of photoinitiators will bring many problems, such as more migratory substances, reduced weather resistance, insufficient cured thickness of the coating film, and increased cost.
Experiments have found that the use of composite photoinitiators in photocuring formulations can effectively overcome the above problems, thereby bringing many advantages. It is particularly important that better curing results can be obtained.
Four commonly used photoinitiators were used in the experiments: 184, 1173, TPO and 819. Chemically they belong to two classes of compounds: α-hydroxy ketones and acyl phosphine oxides.
| English name | Product name | CAS number |
| HCPK | photoinitiator 184 | 947-19-3 |
| HMPP | photoinitiator 1173 | 7473-98-5 |
| TPO | photoinitiator TPO | 75980-60-8 |
| BAPO | photoinitiator 819 | 162881-26-7 |
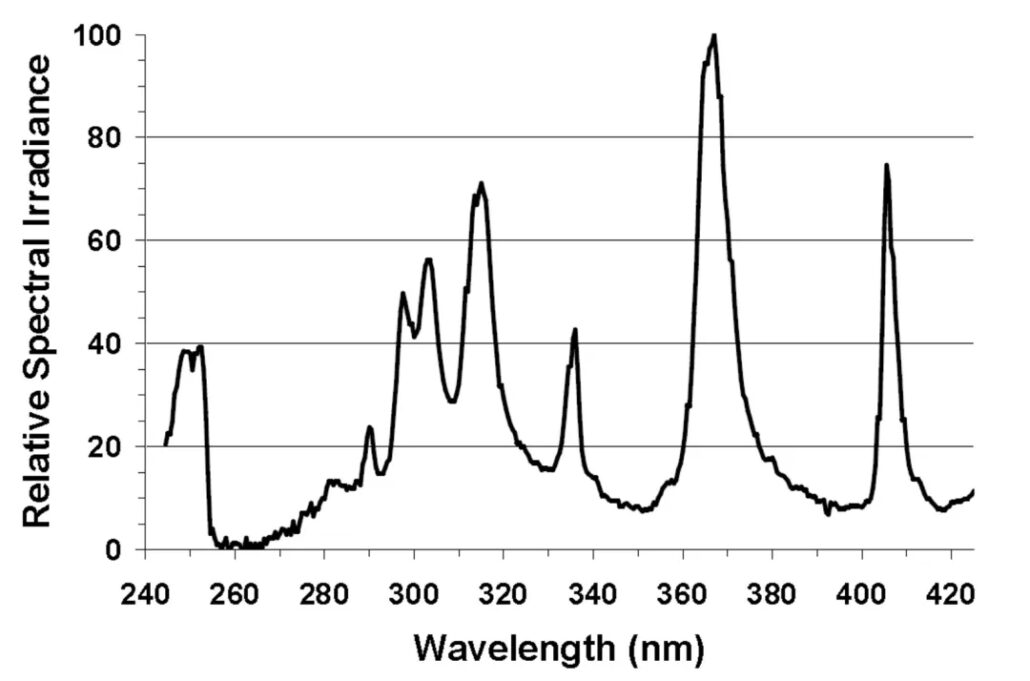
The curing equipment used in the experiment is an Oriel 100-watt mercury lamp (the emission spectrum is shown in Picture 2), and the film thickness is controlled at 50 μm.
The degree of curing was detected by Fourier transform infrared spectroscopy (FTIR) to monitor the change of the characteristic absorption peak of acrylate unsaturated double bond at 810cm-1. The 750-780cm-1 band was also used as the reference peak because it does not change during the entire photocuring process.
The formula for calculating the double bond conversion rate (Reacted Acrylate Unsaturation, RAU) is:
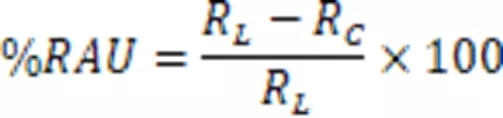
Where RL is the ratio of the acrylate double bond absorption peak to the reference peak in the liquid state; and RC is the ratio of the acrylate double bond absorption peak to the reference peak after UV curing.

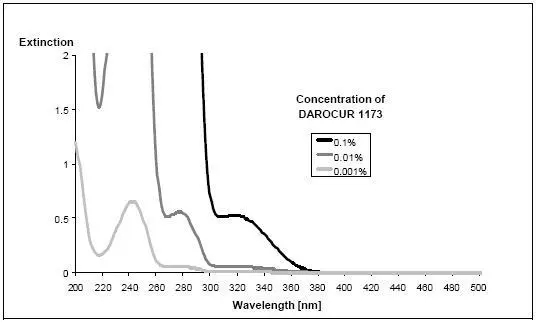

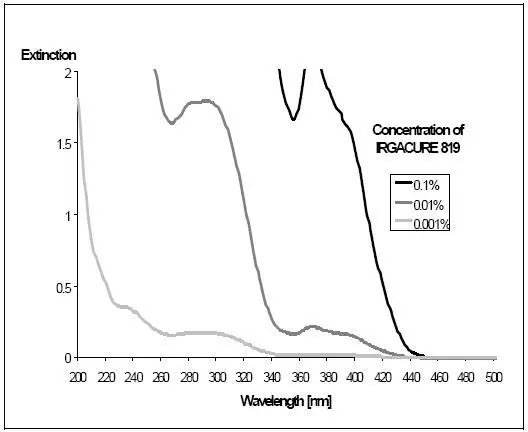
The main absorption of HCPK (photoinitiator 184) is in the wavelength range of 240-250nm, and the absorption peak is in the range of 320-335nm. Another hydroxyketone photoinitiator, HMPP (Darocur 1173), has a similar absorption in the 320-335 nm range with a peak at 265-280 nm. Just using a combination of these two photoinitiators, it is already possible to start to make better use of the output of the UV lamp (Figure 2).
The spectra of TPO and BAPO (photoinitiator 819) are significantly different from the previous two, photoinitiator TPO has a strong absorption in the range of 360-395nm, and BAPO has a stronger absorption in the range of 360-410nm. The addition of the latter two photoinitiators can make better use of the other two main wavelength bands of the mercury lamp at 370 and 408 nm.
In the first experiment, the same amount (weight ratio) of 184 and composite photoinitiator were used for comparison. Under the irradiation of UV light with the same energy of 4.5mJ/cm2, the double bond conversion rate of the formula using 184 is 24.8%, while that of the composite photoinitiator formula is as high as 79.6%.

The second experiment is to use 6% of 184 and composite photoinitiator under the irradiation energy of 4.5mJ/cm2, the former’s double bond conversion rate is 18.9%, and the latter is as high as 67.2%. The difference is very significant.

The third experiment used 4% 184 and 3% composite photoinitiator, respectively, which means that the latter formulation using composite photoinitiator used a lower amount of photoinitiator. Under the same irradiation energy (4.5mJ/cm2), the double bond conversion rate of the former is 50.9%, while that of the latter is 66.8%, which is higher.

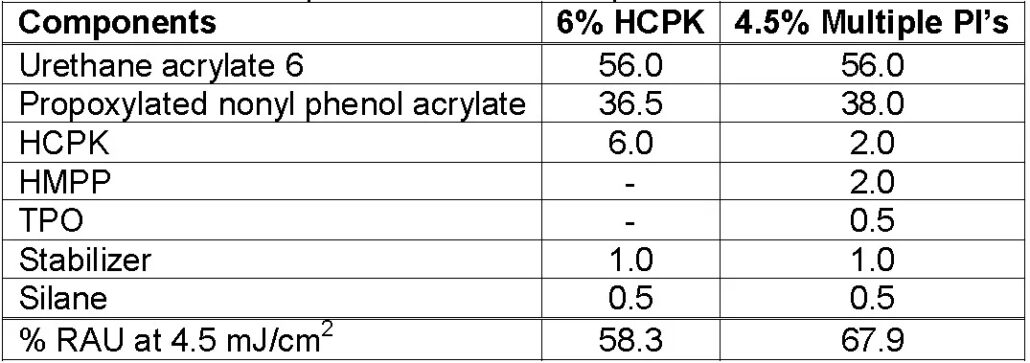
The fourth experiment used 6% 184 and 4.5% composite photoinitiator, respectively. When the radiation energy remains the same (4.5mJ/cm2), the double bond conversion rate of the former is 58.3% and that of the latter is 67.9%. The third and fourth experiments show that the double bond conversion rate can be higher for the formulation of the composite photoinitiator, even with a smaller amount.
The experimental results show that the use of composite photoinitiators can greatly improve the initiation efficiency of photoinitiators. Although the above experiments only compared one photoinitiator ( photoinitiator 184) as a reference object, and the irradiation equipment was only carried out with a mercury lamp, the results can also sufficiently illustrate the advantages of composite photoinitiators.
We know that the use of photoinitiators in the formula is not the better, because too much photoinitiator will absorb ultraviolet light, which greatly affects the penetration efficiency of ultraviolet light during deep curing, thereby affecting the depth of curing.
The use of this composite photoinitiator can not only reduce the cost of formulations, but also achieve better deep curing, reduce photoinitiator residues, and lower costs.
Contact Us Now!
If you need Price, please fill in your contact information in the form below, we will usually contact you within 24 hours. You could also email me info@longchangchemical.com during working hours ( 8:30 am to 6:00 pm UTC+8 Mon.~Sat. ) or use the website live chat to get prompt reply.
