在合成实验中必须了解的 20 种有机反应机理
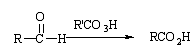
Baeyer-Villiger 反应的反应机理是过酸首先与羰基发生亲核加成反应,然后酮羰基上的一个烃基带着一对电子迁移到-O-O-基团中与羰基碳原子直接相连的氧原子上,同时发生 O-O 键异解。因此,这是一个重排反应
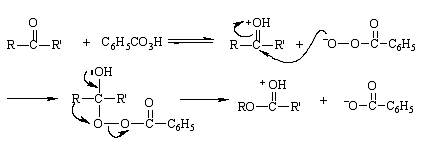
具有光学活性的 3-苯基丁酮与过酸发生反应,重排产物中手性碳原子的浆果保持不变,这表明该反应是分子内重排。
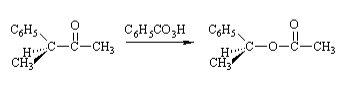
当不对称酮被氧化时,两个基团都可以在重排步骤中迁移,但仍有一定的选择性,迁移能力的顺序如下。
![]()
醛氧化的机理类似,但迁移的是氢负离子,生成的是羧酸。