Magnetic biopolymer microspheres are a novel composite material that combines biological materials and inorganic magnetic materials to form magnetically responsive and biologically active microspheres. Its properties depend on inorganic magnetic materials, biological materials and their interactions method. At present, the most widely used and studied magnetic material is Fe3O4 magnetic nanoparticles. Due to its large specific surface area, good biocompatibility, and high magnetic response, it can achieve rapid separation and targeted movement, so it can be widely used in food and medical care, environmental protection and other fields. Biopolymer materials mainly include chitosan, sodium alginate, gelatin, etc. Among them, chitosan is the most studied biological material due to its own characteristics of good biocompatibility, renewable resources, and biodegradability. A new type of composite material composed of chitosan and magnetic material has both excellent properties, so it has broad application prospects in various fields.

1. Structure and properties of magnetic biopolymer microspheres

The structure of magnetic biopolymer microspheres includes three types: (1) core-shell structure; (2) hybrid structure; (3) multilayer sandwich structure, as shown in Figure 1.
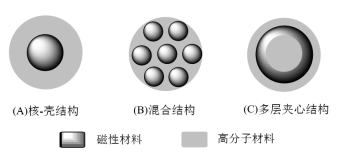
Figure 1 The structure of magnetic biopolymer microspheres

Magnetic biopolymer microspheres have many outstanding properties, making them more suitable for enzyme immobilization. Such as (1) surface area effect. Generally, when the particle size of magnetic biopolymer microspheres reaches the micron or even nanometer level, with the specific surface area increasing, the group density and selective adsorption performance of the microspheres also increases, and the stability of the microspheres increases significantly. (2) Magnetic effect. When the diameter of Fe3O4 crystal is less than 30 nm, it has super para-magnetism, that is, the magnetism is very large under the condition of external magnetic field. When the external magnetic field is removed, its magnetism disappears rapidly, so that the microsphere has magnetic orientation under the condition of external magnetic field and can It is quickly separated from non-magnetic materials, and is not permanently magnetized in a magnetic field, so it does not affect subsequent use. (3) Biocompatibility. In nature, biological materials such as proteins and polysaccharides have biocompatibility, which makes them have important applications in biomedical engineering. (4) Functional base characteristics. Biological materials such as chitosan and sodium alginate have abundant active groups (-OH, -COOH, -NH2), which can be covalently combined with biologically active substances or modified with certain chemical groups.

2. Preparation of magnetic biopolymer microspheres

The preparation of magnetic biopolymer microspheres is divided into two steps. The first step is the preparation of magnetic nanoparticles. The methods currently used to prepare Fe3O4 magnetic nanoparticles mainly include chemical co-precipitation, iron salt thermal decomposition, microemulsion and hydrothermal methods. Among them, the chemical co-precipitation method is simple and convenient to operate, and is the most commonly used preparation method. The principle of the chemical co-precipitation method to synthesize Fe3O4 is to synthesize iron oxide by heating and stirring a mixed salt solution of a certain proportion of Fe2+ and Fe3+ (1:2) under anaerobic conditions and quickly adding lye (ammonia or NaOH). Xu et al. used the chemical co-precipitation method to add 4.34 mmol FeCl2鑘4H2O and 8.67 mmol FeCl3·6H2O, respectively, and heat the system to 85 ℃ under nitrogen. After it was completely dissolved, quickly add 25 mL of concentrated ammonia and add a certain amount of sodium citrate, then the Fe3O4 magnetic nanoparticles is synthesized with good mono dispersity and magnetic responsiveness.

After the preparation of magnetic nanoparticles, it needs to be cross-linked with chitosan to prepare magnetic chitosan microspheres. At present, the synthesis methods of magnetic chitosan microspheres mainly include emulsion cross-linking method, spray drying method, photochemical method and in-situ method. Among them, the emulsion cross-linking method is simpler and most widely used. The emulsion cross-linking method is to uniformly disperse Fe3O4 or magnetic fluid in a mixed liquid containing chitosan, surfactant and oil phase to form a water-in-oil microemulsion system, and then add glutaraldehyde, in the system glutaraldehyde and chitosan will have a cross-linking reaction taking place to generate a Schiff base, and the chitosan will cross linked into a network and then coats Fe3O4 in it (as shown in Figure 2). Jiang et al. used the emulsion cross-linking method, using Span 80, liquid paraffin and glutaraldehyde as the surfactant, dispersant and cross-linking agent, respectively, to synthesize magnetic chitosan microspheres with regular spherical shapes and smooth surfaces.
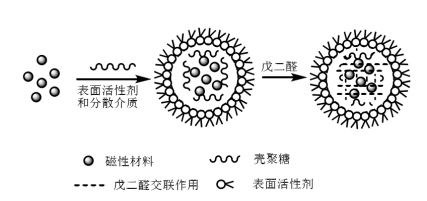
Figure 2 Schematic diagram of the synthesis of magnetic chitosan microspheres by emulsion cross-linking method

3. Enzyme immobilized on magnetic composite microspheres

After the magnetic chitosan microspheres are successfully prepared, the enzyme can be immobilized on the microspheres for use. Since chitosan is rich in active amino groups and hydroxyl groups, it can react with carboxyl groups, amino groups, epoxy groups, bifunctional groups, etc. The magnetic chitosan microspheres are group-modified to meet the needs of different immobilization. The preparation methods of specific immobilized enzymes are introduced below by functional groups.

1) Enzyme immobilized on carboxyl modified magnetic composite microspheres
The carboxyl modified magnetic composite microspheres can be covalently bound to the amino group of the enzyme after being activated by carbodiimide coupling in an aqueous solution, thereby immobilizing the enzyme molecule on the magnetic composite microspheres (Figure 3). Zhu Yihua and others used an improved suspension polymerization method to copolymerize the styrene-treated magnetic fluid and the monomer methyl acrylate through the crosslinking monomer divinylbenzene, and then use alkali hydrolysis to obtain a magnetic composite with good mono dispersity and rich carboxyl groups. The microspheres are used to immobilize lactase after being activated by carbodiimide coupling. The highest activity is about 360 U·g-1, and the cross-linking efficiency of the enzyme is about 20%.
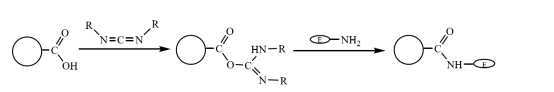
Figure 3 Schematic diagram of preparation of immobilized enzyme on carboxyl modified magnetic composite microspheres

2) Amino modified magnetic composite microspheres immobilized enzyme
After the amino-modified magnetic composite microspheres are coupled with an appropriate amount of glutaraldehyde and activated, they can covalently bind to the amino group on the enzyme, thereby immobilizing the enzyme molecules on the magnetic microspheres (Figure 4). Liu Yu et al. successively prepared monodisperse magnetic SiO2 particles by chemical co-precipitation method and sol-gel method, modified them with amino groups by silane coupling agent, and immobilized laccase with glutaraldehyde as the cross-linking agent. The results showed that the immobilized laccase was kept at a constant temperature of 60 ℃ for 4 hours, and still had 60.9% enzyme activity, and after 10 cycles of use, it still had more than 55% enzyme activity, and its thermal stability and operational stability were obvious improved.
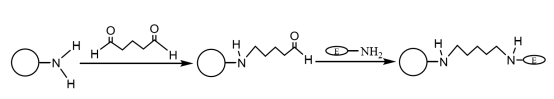
Figure 4 Schematic diagram of preparation of immobilized enzyme on amino-modified magnetic composite microspheres
3) Epoxy modified magnetic composite microspheres immobilized enzyme
Epoxy group is an extremely active group. It can be directly covalently bound to biological groups without modification. Therefore, after the epoxy modified magnetic composite microspheres covalently binding to the amino group on the enzyme to bind the enzyme, the molecules are immobilized on the magnetic microspheres (Figure 5). Yong et al. prepared oleic acid coated magnetic microspheres by suspension polymerization. The hydrophilic epoxy based magnetic microspheres obtained after activation with methanol were used for immobilization of lipase. The retention rate of immobilized enzyme activity is 64.2%, and its stability is significantly improved.
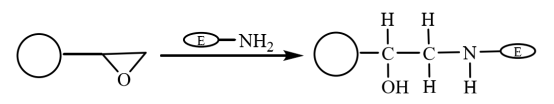
Figure 5 Schematic diagram of preparation of immobilized enzyme on epoxy-based magnetic composite microspheres

4) Enzymes immobilized on magnetic composite microspheres modified by bifunctional groups

On the bifunctional magnetic microspheres, firstly, the carboxyl groups of the enzyme molecule and the amino groups on the microspheres are quickly immobilized on the carrier by ion interaction, and the enzyme immobilized by ion interaction covalently interacts with the epoxy groups on the carrier through its sulfhydryl and amino groups. Make it further fixed (as shown in Figure 6), which has the dual characteristics of rapid fixation by ionic action and firm fixation by covalent bonding. Li Xiutao et al. introduced three random copolymer brushes on the surface of divinylbenzene cross-linked polyacrylic acid microspheres with Fe3O4 nanoparticles dispersed inside, and then used to immobilize penicillin G acylase. The results show that the activity and recovery rate of enzyme activity of the immobilized enzyme with both epoxy group and amino group introduced at the same time are the highest, its immobilization kinetics is better than that of only epoxy-containing magnetic microspheres, and its optimal pH value and temperature stability It is higher than free enzyme, and its enzyme activity retains 70% after repeated use for 10 times.
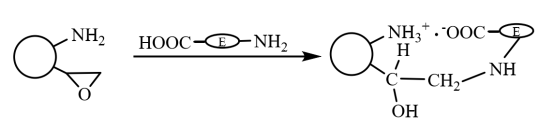
Figure 6 Schematic diagram of preparation of immobilized enzyme on magnetic composite microspheres modified by bifunctional groups

In recent years, although scholars have used different materials to immobilize naringinase, such as natural biopolymer materials such as chitosan, sodium alginate and silk protein, organic compounds such as epoxy resin and polyvinyl alcohol, activated carbon and graphite oxide carbon materials such as ene, and certain research results have been obtained, but in actual debittering applications, there are problems such as poor acid resistance of immobilized enzymes, slow separation from juice or incomplete separation, etc.. Regarding the above problems, the next article will introduce a research work in detail. In this wok, the researchers used a composite material made by chitosan, magnetic Fe3O4 nanoparticles and silica, and modified the composite material with epoxy groups, and then immobilized naringinase on it. This work will provided data basis for further study of naringinase immobilization technology researches.
Contact Us Now!
If you need Price, please fill in your contact information in the form below, we will usually contact you within 24 hours. You could also email me info@longchangchemical.com during working hours ( 8:30 am to 6:00 pm UTC+8 Mon.~Sat. ) or use the website live chat to get prompt reply.
| Compound Glucoamylase | 9032-08-0 |
| Pullulanase | 9075-68-7 |
| Xylanase | 37278-89-0 |
| Cellulase | 9012-54-8 |
| Naringinase | 9068-31-9 |
| β-Amylase | 9000-91-3 |
| Glucose oxidase | 9001-37-0 |
| alpha-Amylase | 9000-90-2 |
| Pectinase | 9032-75-1 |
| Peroxidase | 9003-99-0 |
| Lipase | 9001-62-1 |
| Catalase | 9001-05-2 |
| TANNASE | 9025-71-2 |
| Elastase | 39445-21-1 |
| Urease | 9002-13-5 |
| DEXTRANASE | 9025-70-1 |
| L-Lactic dehydrogenase | 9001-60-9 |
| Dehydrogenase malate | 9001-64-3 |
| Cholesterol oxidase | 9028-76-6 |
