PVC is one of the most widely used polymers in the market today, and it plays an important role in the fields of construction materials and electronic packaging. However, PVC in the external factors (such as heat, oxygen, light and force, etc.) under the action of degradation and cross-linking reaction, resulting in PVC products discoloration, weakening of mechanical properties.PVC heated to 110 ℃ will release HCl gas, so that PVC began to decompose. At present, PVC heat stabilizers are mainly five kinds: lead salt heat stabilizers, metal soap heat stabilizers, organotin heat stabilizers, organic heat stabilizers and rare earth heat stabilizers. Due to lead salts heat stabilizers have heavy metals, serious environmental pollution, is now rarely used. Metal soap type heat stabilizers initially poor anti-color ability, PVC degradation of unstable chlorine atom substitution ability is poor, to meet market demand. Organic tin heat stabilizers have significant stabilizing effect, but some of them are toxic and high cost, which limits its development. Organic heat stabilizers are non-toxic and environmentally friendly, but the stability of PVC heat stabilizers alone is poor. Rare earth heat stabilizers have been studied more, which have the advantages of low toxicity, environmental protection and good thermal stability.
With the development and innovation of science and technology, environmental protection has become an important criterion for heat stabilizers, so nitrogen-containing heterocyclic carboxylic acid heat stabilizers have received wide attention. This is because the heat stabilizer composition does not contain heavy metals, which solves the pollution problem, and it is also an excellent ligand with good thermal stability. By combining the nitrogen-containing heterocyclic carboxylic acid heat stabilizers with rare earth salt solution, a new type of nitrogen-containing heterocyclic carboxylic acid rare earth heat stabilizer can be synthesized. Its advantages are low toxicity, environmental protection, good light transmission, good thermal stability, low cost, high yield and high development potential. Liu Zhaogang et al. used imidazole ⁃ 4,5 ⁃ dicarboxylic acid, sodium hydroxide, lanthanum chloride as raw materials to prepare a nitrogen-containing heterocyclic carboxylic acid rare-earth heat stabilizers, and then used the static heat stabilization experiments and dynamic heat stabilization experiments to conduct a further study, and found that the process of preparation is relatively cumbersome and the reaction time is relatively long, but the product of the thermal stability of the complex is still good. Zhang Ning and others synthesized 8 kinds of lanthanum amino acid heat stabilizers with amino acids, sodium hydroxide and lanthanum nitrate as raw materials, and further studied by static heat stabilization experiments and dynamic heat stabilization experiments, and compared the heat stability of 8 kinds of lanthanum amino acids, and found that the 8 kinds of lanthanum amino acids contained benzene ring and sulfur, which was poisonous and not environmentally friendly, and tryptophan among the 8 kinds of amino acid raw materials contained benzene ring, but the heat stability of lanthanum tryptophan was better than that of 8 kinds of amino acids, and the heat stability of lanthanum tryptophan was better than that of 8 kinds of amino acids. However, the thermal stability of lanthanum tryptophan is the best among the 8 kinds of lanthanum amino acids, and the activation energy of its compound heat stabilizer is better than that of lanthanum tryptophan monomer and commercially available calcium and zinc heat stabilizers, which can enhance the thermal stability of PVC.
In this paper, 2,3⁃PDA was synthesized as a ligand of lanthanum element at pH=6~7. 2,3⁃LPDA was used as the main heat stabilizer of PVC for heat stability study, and then it was compounded with auxiliary heat stabilizers such as calcium stearate, zinc stearate, pentaerythritol, etc., and the heat stability of the compounded system was comparatively analyzed with that of 2,3⁃LPDA and the thermal stabilization of the part of the heat The effects of some heat stabilizers on the plasticizing and mechanical properties of PVC were characterized; finally, the heat stabilization mechanism of 2,3⁃LPDA was investigated.
1
Sample Preparation
2,3⁃LPDA preparation: weigh the appropriate amount of lanthanum oxide into a beaker, add deionized water and stir well; put the beaker into the 60 ℃ water bath heating, stirring the lanthanum oxide aqueous solution with a stirrer, and then use the dropper to slowly add nitric acid solution to the solution of lanthanum oxide is completely dissolved; the pH value of the solution was determined to be 3~4 by pH paper for filtration, the filtrate obtained for the solution of lanthanum nitrate, and poured into a reagent bottle for spare, and its molar value was 0.015 mg/L. The solution was filtered and then poured into a reagent bottle. Reagent bottle spare, its molar concentration determined by EDTA titration; weighing the molar ratio of 3:2 2,3 ⁃ PDA and lanthanum nitrate, the first anhydrous ethanol will be 2,3 ⁃ PDA powder dissolved, and then dilute ammonia to adjust the pH to 6 ~ 7, in constant stirring of the lanthanum nitrate solution is slowly added to the anhydrous ethanol solution of 2,3 ⁃ PDA, and then dilute ammonia to adjust the pH of the system to 6 ~ 7, resulting in a white precipitate, with an electric stirrer. White precipitate, stirred with an electric stirrer for 3 h to make the system fully reactive, and then left to let the precipitate precipitate all precipitated, and then filtered, and then washed the precipitate with anhydrous ethanol for several times; the composite was dried at 50 ℃ to a constant weight, and the resulting product was 2,3 ⁃ LPDA rare-earth heat stabilizers, and then the product was ground into a powder and then packed into a bag for spare use;
Compound heat stabilizer preparation: 2,3 ⁃ LPDA with zinc stearate and pentaerythritol according to different mass ratio of binary and ternary compounding, weighing, grinding and mixing the powder, bagging spare.
2
Results and Discussion
2.1 Characterization of 2,3⁃LPDA
2.1.1 Infrared spectral analysis
Figure 1 shows the FTIR spectra of 2,3⁃PDA and 2,3⁃LPDA. It can be seen that the telescopic vibration peaks of C=N bond in 2,3⁃PDA and 2,3⁃LPDA at 1,540 cm-1 and 1,557 cm-1 , respectively; the telescopic vibration peaks of NO3- on 2,3⁃LPDA at 1,384 cm-1 ; the telescopic vibration peaks of C=O bond on 2,3⁃PDA at 1,752 cm-1 ; the telescopic vibration peaks of C=O bond on 2,3⁃PDA at 1,595 cm-1 and 1,429 cm-1 , respectively; the telescopic vibration peaks of 2,3⁃PDA on 2,3 ⁃PDA at 1,595 cm-1 and 1,429 cm-1 , respectively. at 1,595 cm-1 and 1,429 cm-1 are the antisymmetric and symmetric telescopic vibration peaks of the C=O bond on 2,3⁃LPDA, respectively; 3,266 cm-1 is the telescopic vibration peak of the O-H bond on 2,3⁃PDA; and 934 cm-1 is the characteristically broad peak of -COOH on 2,3⁃PDA, which allows us to determine that carboxylate groups are present; The stretching vibration peak of O-La bond on 2,3⁃LPDA at 652 cm-1, thus indicating that the reaction of 2,3⁃PDA with lanthanum nitrate as a ligand caused the O-H bond in the carboxylate group on 2,3⁃PDA to break the chain and de-H, and formed an O-La bond with the La ion. In summary, it can be determined that the reaction produced 2,3⁃LPDA.
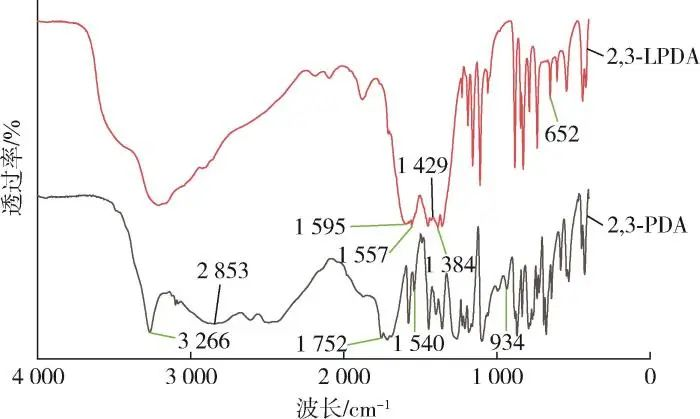
Figure 1 FTIR spectrum of the sample
2.1.2 Elemental and thermal analysis
The elemental content of C, H and N in 2,3⁃LPDA was determined by elemental analysis, and the lanthanum content was determined by EDTA titration. Through Table 1, it can be seen that the relative error of the element H content (mass fraction, the same below) is large because of its small amount, and the actual content of other elements is basically consistent with the theoretical content. Then the number of water of crystallization was calculated by the thermal analysis results in Fig. 2, which led to the molecular formula of 2,3⁃LPDA as La2(C6N2O4)2(NO3)2-3H2O. From the TG curves in Fig. 2, it can be seen that the thermal weight loss of 2,3⁃LPDA was divided into three phases, which were the phases from 50 to 184, 184 to 292, and 292 to 1,000 ℃, respectively. The mass loss rate of the first stage was 5.09 %, and from the mass loss rate, it was deduced that there were three crystalline waters, which was similar to the 6.88 % crystalline water content in the molecular formula La2(C6N2O4)2(NO3)2-3H2O as deduced from Table 1; the DSC curve of the first stage had a heat-absorption peak at 87.1~140.6 ℃, which represented the removal of crystalline water from 2,3 ⁃LPDA. From the TG curves of the second and third stages, it can be seen that 2,3 ⁃LPDA showed a cliff weight loss, with a total mass loss of 58.31% in the second and third stages; in contrast to the DSC curves, there was a 184~292.4 ℃ inspirational peak in its second stage, representing the decomposition of the product. Two exothermic peaks appeared in the third stage, which represented the further decomposition of the products. After 797 ℃, there was no further weight loss, and the TG curve tends to flatten out, and its final residue is La2O3, which accounts for 36.59% of the mass fraction, and the content of La is calculated to be 31.11%, which is similar to the theoretical La content of 31.53% in Table 1. In summary, the molecular formula of the rare-earth heat stabilizer was determined to be La2(C6N2O4)2(NO3)2-3H2O by comparing the data in Table 1 and Figure 2.
Table 1 Elemental analysis results of 2,3⁃LPDA

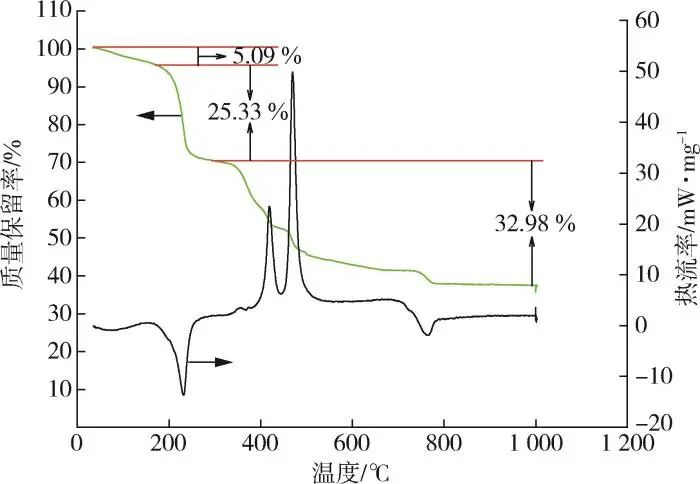
Fig. 2 Thermal analysis curve of 2,3⁃LPDA
2.2 Thermal stability analysis
2.2.1 Single thermal stabilizer
In order to be able to study the thermal stability of 2,3⁃LPDA more deeply, its thermal stability was compared with common thermal stabilizers respectively, and the results are shown in Table 2. According to Table 2, it can be seen that the thermal stabilization time of 2,3⁃LPDA is 30 min, which is 6 times longer than that of 2,3⁃PDA, only shorter than that of lead stearate, and longer than that of other heat stabilizers in the table. In terms of anti-discoloration performance, 2,3⁃LPDA has better initial anti-discoloration performance than 2,3⁃PDA, which indicates that 2,3⁃LPDA has a stronger binding ability with unstable Cl-, and improves the initial anti-discoloration performance of PVC. In terms of long-term anti-discoloration performance, 2,3⁃LPDA is a little weaker than calcium stearate and pentaerythritol in anti-discoloration ability. Except calcium stearate and pentaerythritol, 2,3⁃LPDA has some advantages over other heat stabilizers in anti-discoloration performance, and it has a positive effect on preventing the thermal degradation of PVC.
Table 2 Thermal stability of different heat stabilizers

2.2.2 2,3⁃LPDA compounded with zinc stearate
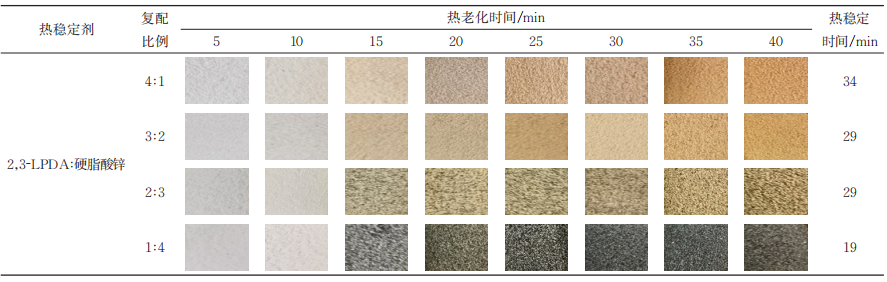
The initial discoloration resistance of 2,3⁃LPDA is slightly worse than that of zinc stearate, but its thermal stabilization time and long-term discoloration resistance are much better than that of zinc stearate. The thermal stability test results of the two complexes are shown in Table 3. From Table 3, it can be seen that the longest thermal stability time was 34 min when the compounding ratio of 2,3⁃LPDA and zinc stearate was 4:1, and it was longer than that when 2,3⁃LPDA was used as a thermal stabilizer alone, and the larger the proportion of 2,3⁃LPDA in the compounding ratio, the longer the thermal stability time. This is because Re3+ and Cl- have strong coordination effects and will more easily coordinate with Cl- decomposed by heat. In terms of anti-discoloration performance, the initial anti-discoloration performance of the compounded heat stabilizers was greatly improved. This is because 2,3 ⁃ LPDA initial inhibition of zinc stearate to produce “zinc burning” phenomenon is obvious, and there is a strong synergistic effect, the compounding of the initial anti-color performance of PVC has been greatly improved. However, from the long-term anti-discoloration performance, 2,3 ⁃ LPDA alone is better than the compounded anti-discoloration performance, and 2,3 ⁃ LPDA in the compounding ratio of the smaller proportion, the “zinc burning” phenomenon will be more obvious, the worse the long-term anti-discoloration, the shorter the heat stabilization time, indicating that the more the proportion of zinc stearate in the compounding ratio of the more, the more the proportion of 2,3 ⁃ LPDA and zinc stearate, and the more the proportion of 2,3 ⁃ LPDA and zinc stearate, the more the proportion of 2,3 ⁃ LPDA and zinc stearate. The shorter the heat stabilization time, indicating that the greater the proportion of zinc stearate in the compounding ratio, the worse the synergistic effect between LPDA and zinc stearate.
Table 3 Thermal stability of zinc stearate compounded systems
2.2.3 Compounding of 2,3⁃LPDA with Pentaerythritol
Pentaerythritol is an auxiliary heat stabilizer with excellent thermal stability, and its short-term and long-term anti-discoloration performance is good. 2,3⁃LPDA and pentaerythritol complexed with the thermal stability is shown in Table 4. From the table, it can be seen that the thermal stability time of the two complexed heat stabilizers are in the range of 31-34 min, and the thermal stability time of 2,3 ⁃LPDA as a heat stabilizer alone was 30 min, which indicates that the thermal stability time of 2,3 ⁃LPDA with pentaerythritol complexed with zinc stearate is 30 min, which indicates that the synergistic effect between 2,3⁃LPDA and zinc stearate is more and more important. 3⁃LPDA and pentaerythritol compounding heat stabilization effect is not obvious, heat stabilization time is only extended by 1-3 min, but it also has a certain synergistic effect. In terms of anti-discoloration performance, the effect of pentaerythritol is very obvious, and the initial anti-discoloration performance of the two composites is better than that of 2,3⁃LPDA alone as a heat stabilizer. From the long-term anti-color performance, the larger the proportion of pentaerythritol in the compound heat stabilizer, the better the long-term anti-color ability, indicating that the compounding of pentaerythritol and 2,3⁃LPDA has good anti-color performance, and pentaerythritol further increases the long-term anti-color performance of 2,3⁃LPDA. Overall, the heat stabilization effect of pentaerythritol is not obvious, but the anti-discoloration property is very prominent. This is due to the alcohol’s ability to change the coloration of PVC when heated, and as the temperature increases, the alcohol undergoes esterification, making it more difficult for the alcohol to precipitate out of the PVC. At the same time, pentaerythritol and lanthanum can be complexed to make up for the broken chains in the molecular chain during the degradation of PVC, thus enhancing the long-term anti-color change ability of PVC.
Table 4 Thermal stability of pentaerythritol complex system
2.2.4 Compounding of 2,3⁃LPDA, zinc stearate and pentaerythritol
The phenomenon of “zinc burn” caused by zinc stearate can be delayed by pentaerythritol. In order to make the heat stabilizers have more excellent anti-color change ability and heat stabilization time, 2,3⁃LPDA, zinc stearate and pentaerythritol were compounded, and their heat stability is shown in Table 5. As shown in Table 5, when the ratio of 2,3⁃LPDA:zinc stearate:pentaerythritol was 2:1:2, the thermal stabilization time was 44 min, which was higher than that of the other two sets of compounding ratios, which fully proved that there was a strong synergistic effect when 2,3⁃LPDA and pentaerythritol accounted for the same proportion. From the view of anti-discoloration performance, due to the addition of pentaerythritol, the anti-discoloration of the compounded system has been greatly improved, and pentaerythritol also slowed down the occurrence of the phenomenon of zinc stearate “zinc burns”, the long-term anti-discoloration of the system after the three compounding has been significantly improved. Comparing Table 2, Table 3 and Table 5, it can be seen that the antidiscoloration performance of the ternary compounding system is greatly improved than that of the binary compounding. This is because, to a certain extent, the auxiliary heat stabilizers of polyols can prevent the occurrence of zinc stearate “zinc burning” phenomenon, and the complex generated by the reaction between pentaerythritol and zinc stearate can effectively weaken the catalytic effect of ZnCl2 on the degradation of PVC.
Table 5 Thermal stability of ternary compounding system
2.3 Analysis of plasticizing properties
In this study, plasticization experiments were carried out on different heat stabilizers to study the effect of heat stabilizers on the dynamic thermal stability of PVC, and the results are shown in Table 6. As can be seen from the table, the plasticization time of the heat stabilizer sample containing zinc stearate compounded with 2,3⁃LPDA was the longest. This is because zinc stearate alone plasticizing, plasticizing performance is very poor, in the end of charging, will immediately appear “zinc burning” phenomenon, the phenomenon will promote the degradation of PVC, can not realize the plasticization; and with 2,3 ⁃ LPDA complex, 2,3 ⁃ LPDA played a role in slowing down the “zinc burning” phenomenon, the effect of the “zinc burning” phenomenon, the effect of the “zinc burning” phenomenon. “After the plasticization peak, zinc stearate started to promote the degradation of PVC, leading to the accelerated decomposition of PVC powder. Although the plasticizing time of the sample containing ternary heat stabilizer is slightly longer than that of the sample containing 2,3 ⁃LPDA, its plasticizing torque and equilibrium torque are lower, which indicates that it can reduce the adhesion between PVC and the processing machinery during the process, thus reducing the energy loss and power consumption.
Table 6 Plasticizing properties of different samples
2.4 Analysis of tensile properties
According to the results of static heat stabilization experiments, the rare earth heat stabilizers with the best heat stabilization performance in each group of experiments were selected for the tensile property test to study the effect of different heat stabilizers on the tensile properties of PVC, and the results are shown in Table 7. The results are shown in Table 7. From the table, it can be seen that the tensile properties of PVC were significantly improved after 2,3⁃LPDA was added to PVC; the difference in tensile properties between the heat stabilizer samples containing 2,3⁃LPDA and zinc stearate and the tensile properties of the samples containing 2,3 ⁃LPDA was large, which may be due to the fact that some of the zinc stearate appeared to have the phenomenon of “zinc burnt” when blending the PVC sheet, which promoted the degradation of PVC. This may be due to the phenomenon of “zinc burning” of some zinc stearate during the mixing of PVC flakes, which promotes the degradation of PVC and reduces the tensile strength of PVC flakes, resulting in the poor mechanical properties of PVC flakes. The last data in Table 7 shows that 2,3⁃LPDA, zinc stearate and pentaerythritol have the best effect on PVC tensile strength enhancement. This may be due to the rare earth elements have many empty orbitals to accept the lone electron of the ligand, while the rare earth metal ions have a large ionic radius, so that it and the organic and inorganic substances in the PVC formula to form a variety of ligands or chelates, increasing the intermolecular interaction force, the PVC played a plasticizing effect, and improve the tensile strength of PVC materials.
Table 7 Mechanical properties of different samples
2.5 Heat stabilization mechanism
The FTIR spectra of 2,3⁃LPDA before and after HCl treatment are shown in Fig. 3. From the figure, it can be seen that the FTIR spectra of 2,3⁃LPDA after HCl treatment shifted the wave number of C=N bond from low wave number to high wave number compared to untreated, and the wave number of C=N bond after treatment was 1,572 cm-1. In addition, the telescopic vibrational peak of NO3- disappeared from the curves after the treatment with HCl due to the fact that the H+ combines with NO3- to form HNO3. However, due to the combination of H+ and NO3- to form HNO3 in the treatment of 2,3⁃LPDA with HCl, it is not possible to determine the thermal stabilization mechanism of 2,3⁃LPDA. HCl treatment of 2,3⁃LPDA, the HCl solution is exothermic, while HNO3 is easily decomposed into H2O, NO2, and O2 under high-temperature conditions, which does not affect the thermal stability properties of 2,3⁃LPDA. In the FTIR spectrum of untreated 2,3⁃LPDA, the stretching vibration peak of O-La bond is at 652 cm-1. After treatment, there were two more characteristic peaks of O-H bond in the FTIR spectra, the telescopic vibration peak of O-H bond at 1,445 cm-1 and the out-of-plane telescopic vibration peak of O-H bond at 1,097 cm-1, and, the spectra reappeared with the – COOH bond’s characteristic peak. From the above, it can be deduced that the O-La bond was broken and the O-H bond was recombined after the treatment, and after the O-La bond was broken, the La ions combined with the Cl ions to form the La-Cl bond, and from the FTIR spectra of the treated 2,3 ⁃LPDA, an additional peak at 1,261 cm-1 was found in the FTIR spectra of 2,3 ⁃LPDA. From the FTIR spectrum of 2,3⁃LPDA after treatment, there is a peak of 1,261 cm-1 , which is the stretching vibration peak of La ⁃Cl bond. It can be seen that 2,3⁃LPDA reacted with HCl to form LaCl3, so it can be known that the stabilizing mechanism of 2,3⁃LPDA is that 2,3⁃LPDA can effectively absorb the HCl gas released during the thermal degradation of PVC and form LaCl3, which can delay the catalytic effect of the thermal degradation of PVC to a certain extent.
