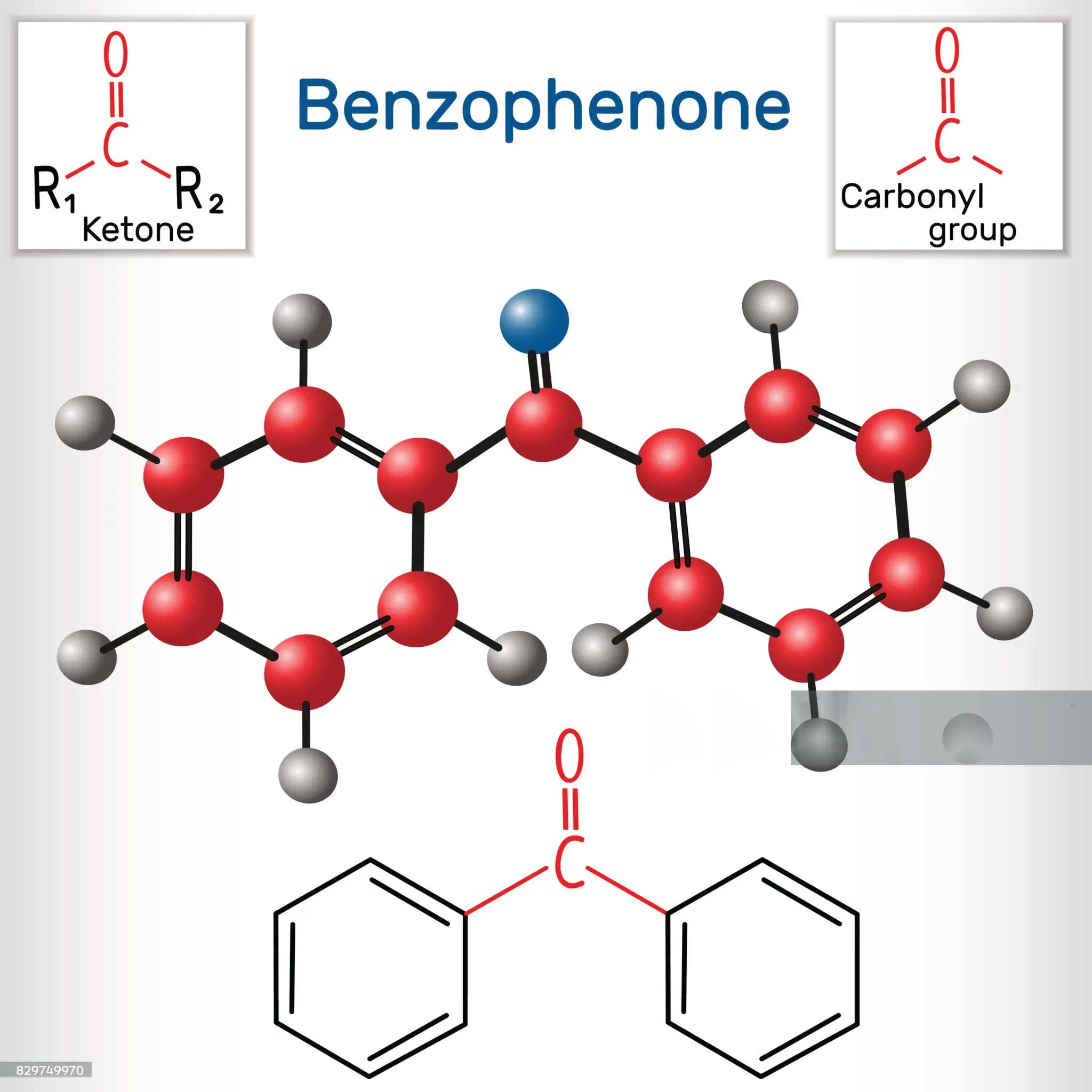
Why is benzophenone insoluble in water? Is benzophenone polar or nonpolar?
Benzophenone is an extremely important photoinitiator in the field of ink manufacturing. However, the question of whether benzophenone is polar or non-polar and its solubility in different solvents has been troubling many practitioners. In this article, we will analyse the polar properties of benzophenone, to answer the question of its insolubility in water but soluble in some alcohol solvents, for the use of photoinitiators benzophenone ink manufacturing plant to provide valuable knowledge and insights.
First, the basis for judgement of the polarity of benzophenone
The molecular structure of benzophenone has a key influence on its polarity. Generally speaking, polar molecules are composed of atoms with different electronegativity, and the molecular structure is asymmetric, and there is a dipole moment. The structure of benzophenone contains a carbonyl group (C=O), which has a certain polarity. However, at the same time, its benzene ring structure has a large non-polar character. In terms of the overall molecule, the volume of the benzene ring and the wider distribution of the electron cloud make benzophenone show a weaker polarity. For example, in some polarity comparison experiments of similar molecules, benzophenone’s polarity performance is significantly lower than that of some strongly polar molecules such as methanol. This is also an important reason for its insolubility in water, because water is a strong polar solvent, according to the principle of ‘similar solubility’, the weak polarity of benzophenone is difficult to dissolve in water.
Solubility analysis of benzophenone in alcohol solvents
When exploring the solubility of benzophenone in alcohol solvents, the situation is more complex. On the one hand, benzophenone itself is insoluble in water because of its weak polarity and inability to form hydrogen bonds with water (due to the absence of hydrogen atoms in its molecule attached to O, N or F). However, in alcoholic solvents, it has been documented to form hydrogen bonds. This is because alcohol molecules (e.g., ethanol, CH₃CH₂OH) have both a non-polar hydrocarbon group portion and a polar hydroxyl (-OH) portion. The carbonyl oxygen atom of benzophenone can form a hydrogen bond with the hydroxyl hydrogen atom of the alcohol molecule, thus enabling solubility to some extent. For example, in the ink blending experiment, when using ethanol as a solvent, benzophenone can be gradually dissolved and uniformly dispersed in the system under the action of stirring, etc., which is the embodiment of hydrogen bonding. However, this solubility is not unlimited, it is affected by a variety of factors such as alcohol molecular structure, concentration and temperature.
Third, the practical significance of the ink manufacturing
For the use of benzophenone manufacturing ink factory, understanding the polarity and solubility of benzophenone has an important practical value. In the ink formulation design, to fully consider the compatibility of benzophenone with other solvents, resins and other components. If an unsuitable solvent is selected, it may result in benzophenone not being adequately dissolved or dispersed, affecting the performance of the ink, such as curing speed and adhesion. For example, if a large number of non-polar solvents are used in the formulation and the matching of benzophenone with polar solvents is neglected, the ink may be precipitated or delaminated during storage. Therefore, reasonable adjustment of the solvent system, according to the characteristics of benzophenone to select the appropriate proportion of polar and non-polar solvents, can optimise the quality of the ink and the production process.
In the ink production process, understanding these properties of benzophenone also helps to control the production conditions. For example, the temperature has an effect on the solubility of benzophenone in the solvent, and an appropriate increase in temperature can enhance its solubility, but it is also necessary to take into account the effect of temperature on the other components and the overall stability of the ink. At the same time, in the procurement of raw materials, the purity and quality testing of benzophenone can also be used to assist in the judgement from the aspect of its solubility, to ensure that the use of benzophenone in line with the production requirements.
From my personal point of view, benzophenone in the solubility of these characteristics does bring a lot of challenges and thinking to the ink manufacturing. In the past some experiments and production practice, I have encountered due to inaccurate grasp of the solubility of benzophenone ink quality problems caused by in-depth study and adjustment of solvent system to be resolved. This also reminds us that in the field of ink manufacturing, the characteristics of each raw material need to be explored in depth in order to produce high-quality products.
In the ink manufacturing process, you can also consider adding some additives to improve the solubility and dispersion of benzophenone. For example, some surfactants can reduce the surface tension of the system and promote better mixing of benzophenone with solvents and other ingredients. At the same time, the selection of production equipment and the optimisation of process parameters need to be combined with the characteristics of benzophenone. For example, the use of efficient mixing equipment and appropriate mixing speed can accelerate the dissolution process of benzophenone and improve production efficiency.
If you want to know more about raw material characteristics and process optimisation in ink manufacturing, welcome to subscribe to our industry newsletter for more professional content.
In conclusion, the polarity and solubility of benzophenone are important factors that cannot be ignored in ink manufacturing. By deeply understanding its properties and applying them to actual production, you can improve the quality and productivity of ink products and win advantages for your company in market competition. Have you ever encountered solubility-related problems in the process of using benzophenone to manufacture inks? Welcome to share in the comments section.
Contact Us Now!
If you need Price, please fill in your contact information in the form below, we will usually contact you within 24 hours. You could also email me info@longchangchemical.com during working hours ( 8:30 am to 6:00 pm UTC+8 Mon.~Sat. ) or use the website live chat to get prompt reply.