20 organic reaction mechanisms that are essential to understand for synthetic experiments
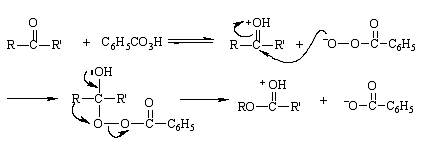
Baeyer-Villiger reaction reaction mechanism peracid first undergoes nucleophilic addition to the carbonyl group, and then a hydrocarbon group on the ketone carbonyl group migrates with a pair of electrons to the oxygen atom directly attached to the carbonyl carbon atom in the -O-O- group, while O-O bond heterolysis occurs. Thus, this is a rearrangement reaction
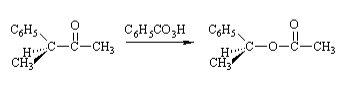
The reaction of an optically active 3–phenylbutanone with peracid, where the berries of the chiral carbon atoms of the rearrangement products remain unchanged, indicates that the reaction is an intramolecular rearrangement.
When an asymmetric ketone is oxidized, both groups can migrate in the rearrangement step, but there is still some selectivity, in the order of their ability to migrate as follows.
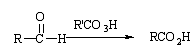
The mechanism of aldehyde oxidation is similar, but the migration is of hydrogen negative ions to give the carboxylic acid.