前面我们提到了芦丁和异槲皮素的转化。在各种转化方法中,产率和纯度最高的转化方法是使用酶催化法,如微生物的 α-L 鼠李糖酶和橙皮甙酶。而α-L-鼠李糖酶通常与β-D-葡萄糖苷酶结合形成柚皮苷酶来发挥催化作用,在早期的研究中,研究人员将 "α-L-鼠李糖酶 "的概念等同于 "柚皮苷酶"。因此,让我们先来介绍一下柚皮苷酶的基本知识。 柚皮苷酶.

柚皮苷酶可以水解柑橘类水果中的柚皮苷和橙皮苷等苦味物质,因此被用于柑橘类果汁的脱苦味处理,柚皮苷酶也因此而得名。柑橘类水果中的主要苦味成分是柚皮苷,柚皮苷酶可通过两个步骤将其降解:第一步,α-L-鼠李糖苷酶将柚皮苷水解为鼠李糖和柚皮苷;第二步,β-D-葡萄糖苷酶进一步将柚皮苷水解为柚皮苷和葡萄糖,但不产生苦味。水解机理如图 1 所示。其中,嘌呤素只含有三分之一的苦味。
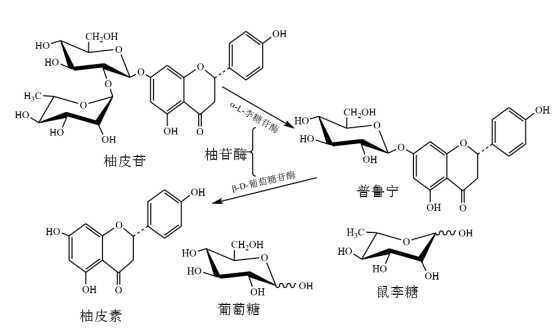
图 1 柚皮苷酶水解柚皮苷的机理
早在 1938 年和 1958 年,Hall 和 Ting 就分别从芹菜种子和柚子叶中获得了柚皮素酶。之后,研究人员又从其他动物和植物中获得了柚皮苷酶。除了动物和植物,柚皮苷酶还广泛存在于微生物中。目前用于研究和工业生产的柚皮苷酶也主要来自微生物。其中,天然真菌是柚皮苷酶的主要来源,如黑曲霉(Aspergillus niger)、米曲霉(Aspergillus oryzae)和青霉(Penicillium)。少量柚皮苷酶来自酵母。还有一些柚皮苷酶来源于细菌,它们的酶性质和应用范围与真菌有很大不同。表 1 列出了一些学者研究的不同来源的柚皮苷酶及其特性。

表 1 不同来源的柚皮苷酶及其特性
| 资料来源 | 菌株 | 基质 | 最佳温度/°C | OptimumpH | 分子量 /kDa |
| 栽种 | 芹菜籽 | 柚皮素 | – | – | – |
| 葡萄柚叶 | 柚皮素 | 50 | 4.0 | – | |
| 椑 | p-NPR、Rutin | – | – | 70 | |
| 动物 | 涡轮增压器 | 柚皮苷、p-NPR、芦丁 | – | 2.8, 4.5~5.0 | – |
| 猪肝 | 薯蓣皂苷 | 42 | 7.0 | 47 | |
| 菌 | 鞘氨单胞菌 R1 | 柚皮素 | 50 | 8.0 | 110 |
| 热微生物 | p-NPR | 70 | 7.9, 5.0~6.9 | 104, 107 | |
| 酸性侧链球菌 | p-NPR、芦丁、橙皮甙 | 50,70 | 5.5, 4.5 | 74, 241 | |
| Brevundimonas sp. | 柚皮素 | 20~37 | 6.0~7.0 | – | |
| 双歧杆菌 | p-NPR、柚皮苷、芦丁、枸橘苷、人参皂苷 | 35 | 6.0 | 100 | |
| 真菌 | 黑曲霉 | 柚皮苷、芦丁、橙皮甙 | 40~60 | 4.0~5.0 | 65 |
| A. kawachii | 柚皮苷、p-NPR、橙皮甙 | 50 | 4.0 | 90 | |
| A. oryzae | 柚皮苷、p-NPR、橙皮甙、新橙皮甙 | 45 | 5.0 | 23 | |
| 十日青霉菌 | 柚皮苷、p-NPR、芦丁 | – | 7.0 | 120 | |
| P. corylopholum | 柚皮苷、芦丁 | 57 | 6.5 | 67 | |
| 酵母 | Pichia angusta | 柚皮苷、芦丁、橙皮甙、槲皮苷 | 40 | 6.0 | 90 |
| 月桂隐球菌 | 柚皮素 | – | – | – | |
| Williopsis californica | 柚皮素 | – | – | – |
对比表 1 中从细菌和真菌中提取的柚皮苷酶的特性可以看出,虽然从真菌中提取的柚皮苷酶的分子量低于细菌,但它更适合在酸性条件下反应,因此适用于柑橘类果汁的脱苦味;而从细菌中提取的柚皮苷酶,糖苷酶最适宜的 pH 环境为中等或弱碱性,反应温度较宽,温度稳定性好。

随着研究的不断深入和具有不同特性的柚皮苷酶的发现,该酶已被广泛应用于医药、食品和化妆品领域。最初的应用是对柑橘类果汁进行脱苦味处理。柚皮苷是柑橘果汁中的主要苦味物质。它在水和果汁中的苦味阈值约为 20 ppm,在某些柑橘类果汁中阈值可达 50 ppm。这表明,当其含量达到百万分之 1.5 时,就会让人感到苦涩。因此,在柑橘等水果的果汁加工过程中,脱苦味处理是一道不可或缺的工序。柚皮苷酶是一种能水解柚皮苷等苦味物质的高效酶,柚皮苷酶能很好地达到脱苦味的目的。黄高玲等用柚皮素酶对琯溪蜜柚果汁进行脱苦味处理,在 60 ℃、pH 值为 3.6 的条件下水解 100 min。果汁的脱盐率可达 97% 以上。Chen Hong 等利用黑曲霉 JMUdb058 通过固态发酵获得柚皮苷酶,并将其用于果汁脱盐。除杂率高达 99.6%,获得了很好的除杂效果。

同时,由于柚皮苷酶中含有α-L-鼠李糖酶,因此可以用来专门生产鼠李糖和嘌呤。鼠李糖是一种甲基戊糖。它可用作药物中间体,合成强心剂和香料呋喃烯醇。它还可以合成香精,同时可用作甜味剂。还可用作肠道渗透试验剂。具有明显的抗癌作用。魏胜华等以柚皮苷酶和酵母静止细胞为催化剂,采用两步生物法转化柚皮苷,制备出质量分数大于 98.5% 的鼠李糖晶体。柚皮苷作为一种黄酮类化合物,在免疫、抗癌、抗病毒、抗氧化等方面具有独特的功能。因此,在食品和医药领域,嘌呤具有重要的应用价值。胡群芳等人采用固态发酵法生产α-L-鼠李糖酶,在适宜条件下对柚皮苷进行生物转化,产品中柚皮苷含量超过95%。

此外,利用柚皮苷酶的反应活性,柚皮苷酶还可进一步用于改善酒的风味。在酒精酿造过程中,各种微生物会产生一些游离的挥发性物质和非挥发性前体物质,α-L-鼠李糖酶首先分解这些非挥发性前体物质,得到单萜类物质β-D-葡萄糖苷,然后β-D-葡萄糖苷酶继续分解释放出单萜类物质,这些物质对提高酒的风味有显著作用。Manzanares 等人利用黑曲霉编码的鼠李糖酶基因 rha A 克隆并在酵母中表达,与其他菌株产生的 β-D 葡萄糖苷酶一起用于葡萄酒发酵,结果葡萄酒中的香味物质显著增加。具体数据如图 2 所示。
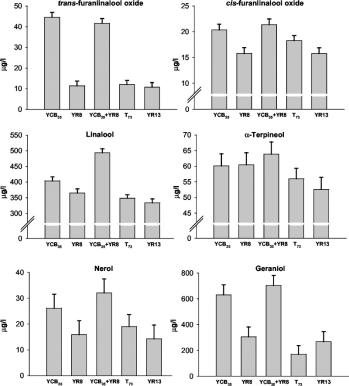
图 2 柚皮苷酶在葡萄酒发酵中的应用
此外,柚皮苷酶还可用于生产抗生素和转化黄酮类化合物。例如,氯多孔菌素是一种脱糖糖肽抗生素,对革兰氏阳性菌有很强的抑制作用。Sankyo 等人发现可以利用柚皮苷酶中鼠李糖酶的活性来合成该抗生素,并发现氯多孔菌素 C 抗生素与内酰胺类抗生素联合使用可有效增强其对葡萄球菌的抗菌效果。Beekwilder 等人从植物乳酸菌中获得鼠李糖酶,并将这种酶用于番茄果肉的发酵。他们发现,这种酶可以去除番茄肉中的鼠李糖,并增强类黄酮的生物转化反应。因此,乳酸菌可提高黄酮类化合物在人体消化系统中的生物转化率。胡福良等人发现,蜂胶黄酮苷可被柚皮苷酶降解合成苷元,从而增强其抗氧化活性。

总之,柚皮苷酶具有非常广阔的应用前景。为了提高柚皮苷酶的重复使用性和稳定性,降低工业生产成本,一般会在反应前将柚皮苷酶固定在载体上。在下一篇文章中,我们将重点介绍酶固定的方法,供大家参考。
立即联系我们!
如果您需要价格,请在下表中填写您的联系信息,我们通常会在 24 小时内与您联系。您也可以给我发电子邮件 info@longchangchemical.com 请在工作时间(UTC+8 周一至周六,上午 8:30 至下午 6:00)或使用网站即时聊天工具获得及时回复。
| 复合葡萄糖淀粉酶 | 9032-08-0 |
| 普鲁兰酶 | 9075-68-7 |
| 木聚糖酶 | 37278-89-0 |
| 纤维素酶 | 9012-54-8 |
| 柚皮苷酶 | 9068-31-9 |
| β-淀粉酶 | 9000-91-3 |
| 葡萄糖氧化酶 | 9001-37-0 |
| α-淀粉酶 | 9000-90-2 |
| 果胶酶 | 9032-75-1 |
| 过氧化物酶 | 9003-99-0 |
| 脂肪酶 | 9001-62-1 |
| 过氧化氢酶 | 9001-05-2 |
| TANNASE | 9025-71-2 |
| 弹性蛋白酶 | 39445-21-1 |
| 尿素酶 | 9002-13-5 |
| DEXTRANASE | 9025-70-1 |
| L 乳酸脱氢酶 | 9001-60-9 |
| 苹果酸脱氢酶 | 9001-64-3 |
| 胆固醇氧化酶 | 9028-76-6 |
