Description
Hydroxymethylfurfural / HMF CAS 67-47-0
5-Hydroxymethylfurfural is an important chemical raw material. It contains an aldehyde group and a hydroxymethyl group in its molecule, and can be used for the synthesis of many useful compounds and new polymer materials, including pharmaceuticals, resinous plastics, diesel fuel additives, etc., through hydrogenation, oxidative dehydrogenation, esterification, halogenation, polymerisation, hydrolysis and other chemical reactions. In particular, biobased PEF polyesters based on furanedicarboxylic acid have demonstrated many properties superior to petroleum-based PET (polyethylene terephthalate).
Standard
| Item | Specification |
| Appearance | Brown and yellow solid |
| Melting point | 28-34 °C |
| Boiling point | 114-116 °C at 1mm Hg |
| Density | 1.243g/mL at 25 °C |
Application:
It can be used in degradable plastic packaging, special functional materials, surfactants, flavours and fragrances and other fine chemicals or pharmaceutical industries.
Package:25kgs/Drum
Storage:
Sensitive to air, light, and heat, with strong moisture absorption.
Sealed and stored at low temperature (<0 ℃).
Contact Us Now!
If you need Price, please fill in your contact information in the form below, we will usually contact you within 24 hours. You could also email me info@longchangchemical.com during working hours ( 8:30 am to 6:00 pm UTC+8 Mon.~Sat. ) or use the website live chat to get prompt reply.
Progress of Research on the Application of Catalysts in Environmental Protection
1. Definition of Environmental Protection Catalysts Environmental protection catalysts refer to the catalysts used to protect and improve the surrounding environment by treating toxic and hazardous substances in a direct or indirect way, making them harmless or reducing them in order to protect and improve the surrounding environment. The scope of environmental protection catalysts can be considered as all catalysts that are beneficial to environmental protection in a broad sense, including catalytic synthesis processes that do not want to or do not produce harmful by-products; in a narrow sense, it is the types of catalysts involved with the improvement of greenhouse effect, ozone layer depletion, the enlargement of the scope of acid rain, and the pollution of water bodies. Environmental catalysts are divided into direct and indirect. For example, the catalyst used to remove nitrogen oxides (NOX) from the exhaust gas belongs to the direct one; and the catalyst used to inhibit NOX production in the combustion process belongs to the indirect one.
2.1 Catalysts for lean-burn vehicles When diesel engines are operated under lean-burn conditions, the air-fuel ratio (ratio of air to fuel) of petrol engines is greater than 17:1, or even higher. At this time, the engine power performance can be greatly improved, reducing the emissions of CO, hydrocarbons, and CO2, however, NOx emissions are greatly increased. For the currently popular three-effect precious metal catalysts, such a high air-fuel ratio is beyond the normal operating range, so it cannot effectively improve the reduction of NOx. Therefore, new automotive catalysts should be developed that can improve NOx conversion under lean conditions, and the catalytic reduction of NOx under lean conditions has attracted the interest of researchers. Once this catalyst is successfully researched, it will be widely used in vehicles with diesel engines and oil-poor petrol engines.
2.2 Research on flue gas desulphurisation The best method for flue gas desulphurisation is the selective catalytic reduction of SO2 to elemental sulphur. This method not only eliminates the source of SO2 pollution in the flue gas, but also recovers the product, i.e. solid elemental sulphur, which is not only easy to transport but also can be reused. At present, most of the methods of selective catalytic reduction of SO2 to elemental sulphur are in the research stage. The problems are the interference of excess oxygen in the flue gas to the reduction process and the poisoning of the catalyst.
2.3 Catalytic oxidation treatment of high-concentration non-degradable organic wastewater With the development of pharmaceutical, chemical and dye industries, there are more and more high-concentration non-degradable wastewater, which is characterised by high toxicity of pollutants, high concentration of pollutants, difficult to be biodegraded, and high content of inorganic salts. One of the most effective methods to treat such wastewater is chemical oxidation. At present, high efficiency wet catalytic oxidation technology is a popular research topic. This method can directly oxidise the organic pollutants in the water or oxidise the large molecule organic pollutants in the water into small molecule organic pollutants, in order to improve the biochemistry of wastewater. With biochemical treatment can better remove organic pollutants in the water. This method is commonly used to increase the catalytic oxidation of organic pollutants oxidants can be used: air, hydrogen peroxide, ozone, sodium hypochlorite and chlorine dioxide and other oxidants. The key to this method is the development of highly efficient non-homogeneous oxidation catalysts.
2.4 Types of environmental protection catalysts and the use of the current situation There are many kinds of environmental problems on the earth, and the problems that need to be solved urgently at present are: the greenhouse effect, the destruction of the ozone layer, the expansion of the scope of acid rain, the emission of heavy metals and other environmental pollutants, the reduction of tropical rain forests and desertification of the soil, etc. The first three of them are the most important problems in the world. The first three of these problems are caused by chemical substances emitted into the atmosphere. For example, carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) are all related to the greenhouse effect, Freon and N2O destroy the ozone layer, and sulphur dioxide (SO2) and NOX are the main factors in the formation of acid rain and photochemical smog, which can be eliminated or reduced mainly through chemical methods. Due to the low amount of reactants involved in the emission process of the above pollutants, the reaction temperature is either too high or too low, and the contact time between the reactants and the catalyst is particularly short, etc., the environmental catalysts, compared with the catalysts used in other chemical reactions, are more difficult to produce and have higher requirements on the activity, selectivity and durability of the catalysts.
2.5 New Environmental Protection Catalysts
2.5.1 Silicate Materials Natural clay such as montmorillonite has a molecular sieve-like structure and is a catalyst carrier and a good adsorbent for treating heavy metal ions in sewage. It is widely used as the carrier of environmental protection catalysts such as automobile exhaust purification, flue gas desulphurisation, denitrification and catalytic combustion of organic waste gas.
2.5.2 TiO2 is an N-type semiconductor with good photosensitive conductivity, often used as a catalyst carrier. Now TiO2 is widely used as photocatalyst and electrode catalyst. Self-cleaning glass, tiles, furniture and curtain cloth coated with active TiO2 automatically catalyses and purifies indoor air under the irradiation of sunlight and light.
2.5.3 Biocatalytic process is usually based on non-toxic and harmless biological materials as raw materials, which can be reacted at room temperature and pressure, and the process is simple. Biocatalysts are ideal green catalysts because of their high conversion rate, high specificity, low by-products and repeated use. 2.5.4 Room temperature ionic liquid can be used as both acid catalyst and a green solvent. With the advantages of easy production, low toxicity, low price, non-combustible, adjustable performance, etc., it is predicted to be an environmentally friendly catalyst with the potential to cause a revolution in the chemical industry and good prospects for industrial application.


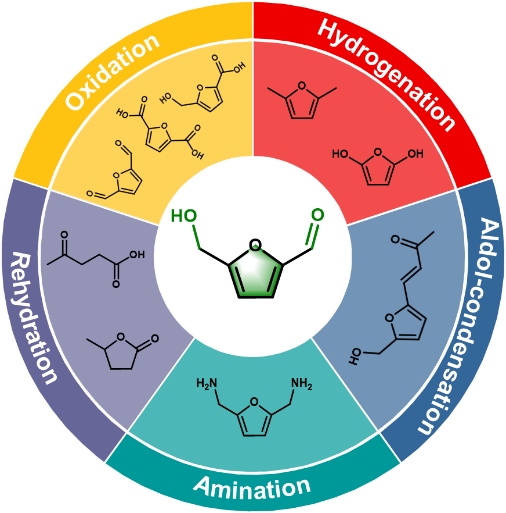 Background
Massive consumption of fossil fuels and growing environmental concerns are forcing a search for more sustainable energy resources. Lignocellulosic biomass is a widely available inedible carbon resource in the world that can be converted into renewable energy and high-value chemicals, and biomass-based chemicals can replace the vast majority of petrochemicals. Among them, the catalytic conversion of biomass-derived 5-hydroxymethylfurfural (HMF) platform compounds has been a popular area for high-value utilisation of lignocellulosic biomass in recent years.HMF has multiple functional groups and is prone to multiple side reactions during the conversion process, which affects the quality of chemical products. Therefore, the design and preparation of efficient green catalytic systems to convert HMF into a variety of high value-added chemicals, liquid fuels and additives by selectively breaking/functionalizing the specific functional groups of HMF is the key to realize the high value-added use of HMF.
Read more
HMF oxidation Firstly, the authors summarised the main products generated by HMF oxidation and mainly discussed three HMF oxidation products 2,5 dicarbonylfuran (DFF), 5-hydroxymethyl-2 furan carboxylic acid (HMFCA) and 2,5 dicarboxylic acid furan (FDCA). The authors systematically introduced the catalyst systems for the selective oxidation of HMF for the preparation of the above three major products, discussing the effects of noble and non-precious metal catalysts, and reaction solvent acidity and alkalinity on the selectivity of the products, respectively. Secondly, the reaction mechanisms of HMF for the preparation of DFF, HMFCA and FDCA were summarised. In addition, the large-scale production of high-value chemicals prepared from HMF oxidation is partially discussed, especially the preparation of FDCA, and its techno-economic analysis is presented.
Background
Massive consumption of fossil fuels and growing environmental concerns are forcing a search for more sustainable energy resources. Lignocellulosic biomass is a widely available inedible carbon resource in the world that can be converted into renewable energy and high-value chemicals, and biomass-based chemicals can replace the vast majority of petrochemicals. Among them, the catalytic conversion of biomass-derived 5-hydroxymethylfurfural (HMF) platform compounds has been a popular area for high-value utilisation of lignocellulosic biomass in recent years.HMF has multiple functional groups and is prone to multiple side reactions during the conversion process, which affects the quality of chemical products. Therefore, the design and preparation of efficient green catalytic systems to convert HMF into a variety of high value-added chemicals, liquid fuels and additives by selectively breaking/functionalizing the specific functional groups of HMF is the key to realize the high value-added use of HMF.
Read more
HMF oxidation Firstly, the authors summarised the main products generated by HMF oxidation and mainly discussed three HMF oxidation products 2,5 dicarbonylfuran (DFF), 5-hydroxymethyl-2 furan carboxylic acid (HMFCA) and 2,5 dicarboxylic acid furan (FDCA). The authors systematically introduced the catalyst systems for the selective oxidation of HMF for the preparation of the above three major products, discussing the effects of noble and non-precious metal catalysts, and reaction solvent acidity and alkalinity on the selectivity of the products, respectively. Secondly, the reaction mechanisms of HMF for the preparation of DFF, HMFCA and FDCA were summarised. In addition, the large-scale production of high-value chemicals prepared from HMF oxidation is partially discussed, especially the preparation of FDCA, and its techno-economic analysis is presented.
 Fig. 1 HMF can be oxidized into many compounds that are obtained from petroleum sources
Fig. 1 HMF can be oxidized into many compounds that are obtained from petroleum sources
 Fig. 2 Possible oxidation mechanism of HMF to DFF over ZnFe1.65Ru0.35O4. (Energy & Fuels, 2017, 31, 533-541.)
Fig. 2 Possible oxidation mechanism of HMF to DFF over ZnFe1.65Ru0.35O4. (Energy & Fuels, 2017, 31, 533-541.)
 Fig. 3 Oxidation mechanism of HMF to HMFCA over AgO catalyst in the presence of H2O2. (ACS Sustain. Chem. Eng., 2020, 8, 8486-8495.)
Fig. 3 Oxidation mechanism of HMF to HMFCA over AgO catalyst in the presence of H2O2. (ACS Sustain. Chem. Eng., 2020, 8, 8486-8495.)
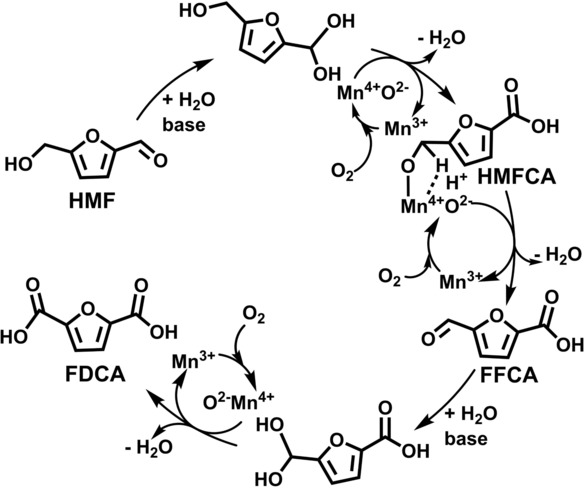 Fig. 4 Oxidation mechanism of HMF to FDCA over holey Mn2O3 nanoflakes. (ChemSusChem, 2020, 13, 548-555)
HMF Hydrogenation Firstly, it is summarised that HFM can be hydrogenated to obtain a wide range of high-value chemicals, which can be used as fuels or fuel additives and have properties not inferior to petrochemicals. It focuses on the preparation of DHMF, DHMTHF, and DMF by HMF hydrogenation.Then, it summarises the effects of noble metal catalysts, non-precious metal catalysts, bimetallic catalysts, the nature of the carriers, and the effect of solvents on the HMF hydrogenation products. Due to the growing maturity of HMF to DMF, large-scale preparation of biomass-based DMF is possible. In this paper, examples of large-scale preparation of DMF are also presented and their techno-economics are analysed, indicating that biomass-based DMF has a good prospect for industrial application.
Fig. 4 Oxidation mechanism of HMF to FDCA over holey Mn2O3 nanoflakes. (ChemSusChem, 2020, 13, 548-555)
HMF Hydrogenation Firstly, it is summarised that HFM can be hydrogenated to obtain a wide range of high-value chemicals, which can be used as fuels or fuel additives and have properties not inferior to petrochemicals. It focuses on the preparation of DHMF, DHMTHF, and DMF by HMF hydrogenation.Then, it summarises the effects of noble metal catalysts, non-precious metal catalysts, bimetallic catalysts, the nature of the carriers, and the effect of solvents on the HMF hydrogenation products. Due to the growing maturity of HMF to DMF, large-scale preparation of biomass-based DMF is possible. In this paper, examples of large-scale preparation of DMF are also presented and their techno-economics are analysed, indicating that biomass-based DMF has a good prospect for industrial application.
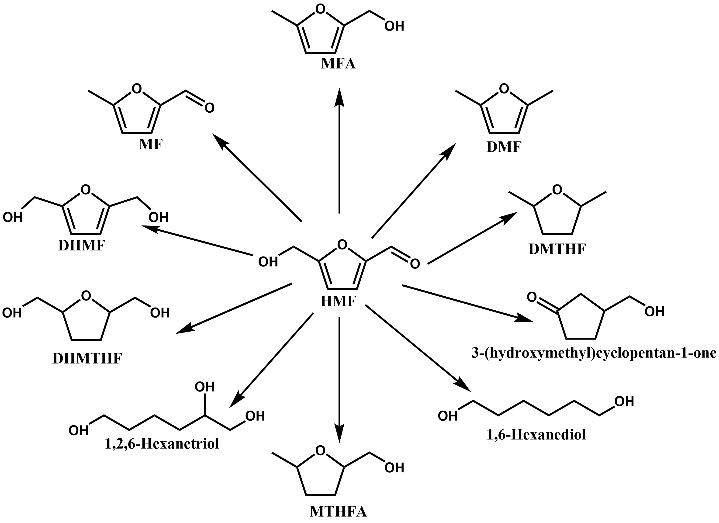 Fig. 5 A number of chemicals generated from the selective hydrogenation or hydrogenolysis of HMF.
Hydroxyaldol condensation In order to increase the carbon chain of HMF and improve the value of HMF, the aldehyde group of HMF can be used to increase the chain by hydroxyaldol condensation, and then further hydrodeoxygenation to obtain high-quality alkane fuels. This paper introduces the types of hydroxyaldol condensation that can occur in HMF, and takes the hydroxyaldol condensation reaction between HMF and acetone as an example to synthesise C9, C12 and C15 alkanes. The catalysts for hydroxyaldol condensation of HMF are also summarised.
Fig. 5 A number of chemicals generated from the selective hydrogenation or hydrogenolysis of HMF.
Hydroxyaldol condensation In order to increase the carbon chain of HMF and improve the value of HMF, the aldehyde group of HMF can be used to increase the chain by hydroxyaldol condensation, and then further hydrodeoxygenation to obtain high-quality alkane fuels. This paper introduces the types of hydroxyaldol condensation that can occur in HMF, and takes the hydroxyaldol condensation reaction between HMF and acetone as an example to synthesise C9, C12 and C15 alkanes. The catalysts for hydroxyaldol condensation of HMF are also summarised.
 Fig. 6 Aldol condensation with acetone followed by hydrogenation and hydrogenolysis.
Rehydration Reactions This paper firstly describes the mechanism of the rehydration reaction that occurs in HMF to produce acetylpropionic acid and formic acid. Acetylpropionic acid (LA) is another important biomass platform molecule, the catalytic system for the conversion of HMF to LA is mainly introduced, and the pathways for the conversion of LA to other important chemicals are briefly summarised.GVL is also an important biomass platform molecule, which can be obtained by the conversion of HMF, and the pathways for the conversion of GVL to other chemicals are briefly summarised as well.
Fig. 6 Aldol condensation with acetone followed by hydrogenation and hydrogenolysis.
Rehydration Reactions This paper firstly describes the mechanism of the rehydration reaction that occurs in HMF to produce acetylpropionic acid and formic acid. Acetylpropionic acid (LA) is another important biomass platform molecule, the catalytic system for the conversion of HMF to LA is mainly introduced, and the pathways for the conversion of LA to other important chemicals are briefly summarised.GVL is also an important biomass platform molecule, which can be obtained by the conversion of HMF, and the pathways for the conversion of GVL to other chemicals are briefly summarised as well.
 7 Horvat's mechanism for HMF decomposition in presence of acid. (Energy & Fuels, 2011, 25, 4745-4755.)
Ammoniation The ammoniated products of HMF can be used as important intermediates in chemical and pharmaceutical fields. In this paper, a systematic overview of the ammonification reaction of HMF is given, with special reference to the catalyst types in the ammonification reaction of HMF and the effect of different amines. In addition, the authors summarise the recent progress of polymerisation, etherification and decarboxylation reactions of HMF.
7 Horvat's mechanism for HMF decomposition in presence of acid. (Energy & Fuels, 2011, 25, 4745-4755.)
Ammoniation The ammoniated products of HMF can be used as important intermediates in chemical and pharmaceutical fields. In this paper, a systematic overview of the ammonification reaction of HMF is given, with special reference to the catalyst types in the ammonification reaction of HMF and the effect of different amines. In addition, the authors summarise the recent progress of polymerisation, etherification and decarboxylation reactions of HMF.
 7 Horvat's mechanism for HMF decomposition in presence of acid. (Energy & Fuels, 2011, 25, 4745-4755.)
Ammoniation The ammoniated products of HMF can be used as important intermediates in chemical and pharmaceutical fields. In this paper, a systematic overview of the ammonification reaction of HMF is given, with special reference to the catalyst types in the ammonification reaction of HMF and the effect of different amines. In addition, the authors summarise the recent progress of polymerisation, etherification and decarboxylation reactions of HMF.
7 Horvat's mechanism for HMF decomposition in presence of acid. (Energy & Fuels, 2011, 25, 4745-4755.)
Ammoniation The ammoniated products of HMF can be used as important intermediates in chemical and pharmaceutical fields. In this paper, a systematic overview of the ammonification reaction of HMF is given, with special reference to the catalyst types in the ammonification reaction of HMF and the effect of different amines. In addition, the authors summarise the recent progress of polymerisation, etherification and decarboxylation reactions of HMF. 


Reviews
There are no reviews yet.