The composition of super glue 502-ethyl cyanoacrylate
Super glue is a common commodity in daily life and has a wide range of uses. In the subconscious, 502 glue seems to have the super ability to glue anything instantly, after all, the English name of super glue, “super glue”, reveals a “king of breath”. When we were young, we might all have the horrible experience of being stuck with 502 glue inadvertently. We can’t help wondering what exactly is the composition of 502 glue and why it has super bonding ability? Is there a way to quickly remove 502 glue adhesion? ?
From an unexpected discovery
Eastman Kodak (Eastman Kodak) is a world-renowned photographic equipment company. What’s interesting is that superglue was born here by accident. In 1942, Dr. Harry Coover, who worked for Kodak, tried to find an optical plastic that could be used in lenses. Although the synthesized cyanoacrylates worked well, they were extremely viscous. Strong, Dr. Coover reluctantly gave up and continued to explore. When the time came to 1951, Dr. Kuffer set out to search for heat-resistant acrylate polymers to be used in jet aircraft canopies. One day, a student had to break the container to take out the product after synthesizing ethyl cyanoacrylate. This compound is too viscous, but it will only stick to other objects when it comes in contact with it.
Fortunately, Dr. Kuffer did not ignore this substance as easily as he did a few years ago. He realized that this may be an unprecedented adhesive with strong application prospects and commercial value. In 1958, this adhesive was introduced to the market by Kodak under the trade name Eastman 910, and was subsequently renamed as the well-known Super Glue. With this product, Dr. Cuffer himself was awarded the National Medal of Science and Technology Innovation in 2010 (National Medal for Innovation). Medal of Technology and Innovation).
How does super glue work?
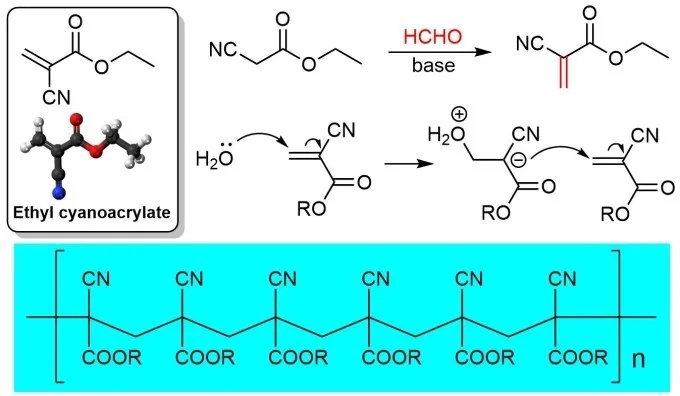
The cyanoacrylate mentioned above is actually a general term for a class of compounds. The ingredients in different super glues are actually different. For example, the glue for medical adhesive wounds contains octyl cyanoacrylate, which is currently the composition of most super glues. It is Ethyl cyanoacrylate (ECA), and a small part is a methyl ester derivative. From the perspective of synthetic chemistry, this type of compound is very easy to prepare. Using the classic Knovenagel reaction, large-scale industrial production can be easily achieved. However, the magic of this uncomplicated molecule is not only simple in synthesis, but also in its strong adhesion.
Many people may have wondered why super glues can be stored stably in the container in which they are stored since they are extremely viscous. The reason is that if the super glue wants to play a binding effect, it must rely on a certain “initiator”, which is actually an inconspicuous trace of water molecules in the air. The basic component of super glue is cyanoacrylate monomer. Once it comes into contact with water vapor, it will initiate anionic polymerization and finally form a high-strength polymer. As the polymerization process releases heat, the ingredients and solvents in the glue will accelerate Evaporation, so we often smell unpleasant odors during use. In order to obtain a better adhesion effect in life, we often think that the more glue, the stronger the adhesion. From the point of view of the mechanism of glue, it is actually unreasonable. A large amount of glue will cause the adhesion part to thicken, which will affect its adhesion. The combination of water vapor will also affect the strength of the final polymer.
Other uses
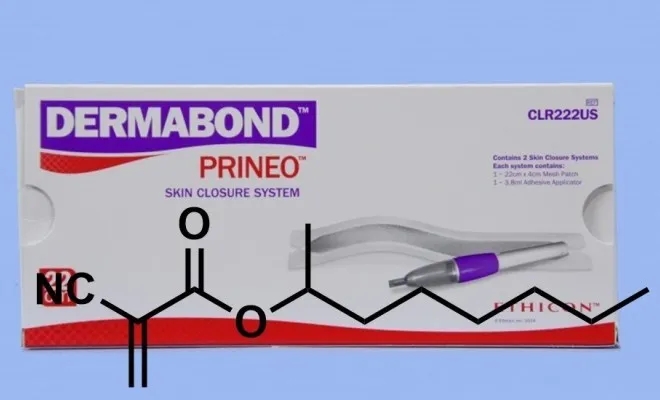
In addition to the application in the field of super glue, the improved cyanoacrylate also has important applications in the medical field. During the Vietnam War, U.S. military doctors used super glue to sew wounds for wounded soldiers. However, the cyanoacrylates used at the time did not meet the medical conditions. After use, they often caused skin sensitivity. What’s more, these short-chain carboxylates were more serious. It is easier to disaggregate, and fragmented monomers are not conducive to wound healing and sometimes cause wound infection. Fortunately, in 1998, people developed octyl cyanoacrylate. Long-chain carboxylic acid esters are not easy to depolymerize and have better affinity for human tissues. Later, this glue was named Dermabond and became a special wound suture glue for human skin.
Another use of cyanoacrylate is in criminal investigation. The police can use super-glue fuming to reveal potential fingerprints. The general process is as follows: First, place the fingerprint-bearing object in a heated and In an airtight container, put the super glue into the container. The cyanoacrylate is heated to evaporate, and at the same time it is circulated and dispersed in the container with the help of a fan. The residual substances on the fingerprints such as amino acids, glucose and moisture will be mixed with the gas. The modified glue undergoes a reaction similar to the gradual polymerization of monomers during bonding, and fingerprints will appear immediately. It is said that this method was used in criminal investigations in Japan as early as 1978.


Fingerprint display (two drops are water and super glue)
Concluding remarks
Cyanoacrylate series adhesives have the characteristics of simple composition, room temperature curing, fast bonding speed and high strength. They have strong adhesion to metals, plastics, rubber, ceramics and even human tissues. In recent years, with the development of bonding technology, the research and application of medical adhesives have also made great progress. At the same time, due to the continuous improvement of people’s living standards, consumers have also put forward new and higher requirements for adhesives. In the future, adhesives with low odor, small bonding surface and environmentally friendly adhesives will be new development directions. Finally, back to the question at the beginning of the article, how to quickly remove the adhesion of 502 glue?-The most effective thing is of course the “similar compatibility” effect of acetone. Because pure acetone is not commonly regulated in life, many washes Nail water contains acetone, which is a good choice for removing and curing 502.
How to remove the cyanoacrylate adhesive from the skin?
If eyelids or lips are stuck, consult a doctor. If you have tied your fingers tightly, soak them in warm soapy water and gently roll them together (like rolling a pencil between your fingers). Don’t separate them, because you are likely to peel the skin from one finger.
How to remove cyanoacrylate adhesive from plastic?
If you can spend some time with water, this may be your best choice. Both acetone and CA Solvent 100 can apply pressure to and turbid a variety of plastics. It is best to test the solvent on the plastic before use.
How to remove cyanoacrylate adhesive from glass?
The good news here is that the cyanoacrylate adhesive does not bond to glass very well, so you can usually scrape it off with a razor. If the bond looks strong, you can use water, acetone or CA Solvent 100, or just wait a few days (the bond will degrade) and try again.
How to remove cyanoacrylate adhesive from metal?
Unlike glass, cyanoacrylate has a strong bond with metal. The good news here is that metals can withstand high temperatures, so boiling water or room temperature acetone or CA Solvent 100 can be used.
