How to solve the problem of large amount of waste salt caused by acid binders?
1, antioxidant 3052 is a multi-functional main antioxidant, with the traditional phenolic antioxidant mechanism, compared with the antioxidant 3052 can be stabilized by its own bifunctional stabilization mechanism, capture the macromolecular free radicals quickly stabilized to phenol oxygen radicals. Because of its outstanding synergistic effect, and can stably reduce the aging of resin, it plays a more prominent role in the production of rubber, synthetic resin process, so that the polymer material is more durable. The biggest feature of antioxidant 3052 is its ability to resist thermal oxygen at high temperature, which is a necessary component in the additives of polymer materials, especially in the conditions of low oxygen content can play a greater role.
2, antioxidant 3052 is a new type of antioxidant, with high efficiency to prevent polymer thermo-oxidative aging ability, because its molecule has phenolic hydroxyl and acrylate group two active groups, can effectively control the butadiene homopolymer and copolymer gel molding, especially in the high-temperature processing of the oxygen content of the conditions of the lower its protective effect appears more prominent. Therefore, it has high antioxidant capacity, non-discoloration, low volatility and excellent resistance to extraction performance, its application field is also extremely wide, can be applied to the synthetic rubber industry, hot melt adhesives, elastomers, packaging materials in contact with food and drugs and other fields, but also in the field of auxiliary industry in the more important kind of products.
3, antioxidant 3052 and sulfur ester antioxidants and phosphite antioxidants have a good synergistic effect when used in conjunction, usually also used in conjunction with hindered amine antioxidants and benzotriazole UV absorbers. Compared with the traditional bisphenol type antioxidant 2246, antioxidant 3052 has a higher melting point and can withstand higher temperatures.
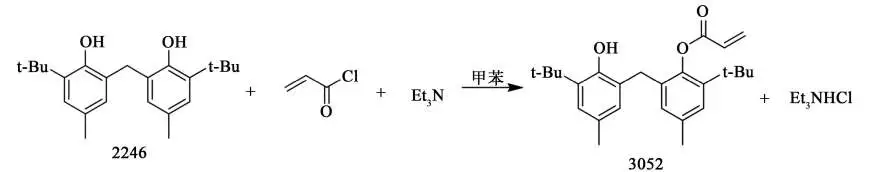
4, there are currently a total of two methods for synthesizing antioxidant 3052. They are step-by-step synthesis and one-pot method synthesis. In the step-by-step synthesis is divided into two a kind of organic acid, chlorine phosphorus oxide synthesis of chlorine chloride, and then prepared by the chlorine chloride and bisphenol into the antioxidant, this preparation and synthesis method was first developed by the Japanese Sumitomo Chemical Company, and was later widely used. Another method is to prepare antioxidants from bisphenol, chloric acid chloride and organic bases, which was developed by Sumitomo Chemical to synthesize such antioxidants. The one-pot synthesis involves the preparation of chloride compounds from bisphenol, carboxylic acid and solid phosgene, with organic bases as catalysts. At the end of the reaction, without separation, a certain amount of organic solvent is added directly, and then a bisphenol solution containing the stool solvent is added dropwise, and the reaction is continued at a certain temperature for a certain period of time. At the end of the reaction, the precipitate is removed by filtration under reduced pressure, and the solvent is removed by distillation at atmospheric pressure. Precipitation of crystals, the crystalline material by recrystallization, filtration and drying of white solid, that is, a new type of bisphenol monoester antioxidant products.
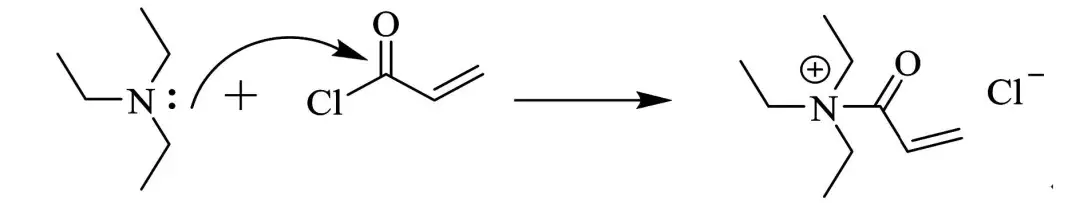
5, in the traditional preparation process, you need to prepare the intermediate 2,2′-methylene bis (4-methyl-6-tert-butylphenol), originally phenol hydroxyl and aromatic ring to form a conjugation effect reduces the oxygen atom on the electron cloud density, so phenol hydroxyl nucleophilic performance is weak, can not be esterified with carboxylic acids directly, when access to push the electron group in the benzene ring further reduces the phenol hydroxyl nucleophilic performance, and at the same time when in the At the same time, when accessing a large group on the benzene ring of 2-tert-butyl-4-methylphenol, a spatial effect will further reduce the activity of phenolic hydroxyl group, leading to the preparation process is more difficult. Moreover, in the conventional process of synthesizing antioxidant 3052, no matter which method is bypassed by using chloric acid chloride as raw material, organic alkali as acid-binding agent and catalyst, organic alkali becomes organic salt to become solid waste, in addition to the conventional method of preparation will produce hydrochloric acid, need to add triethylamine to remove the hydrochloric acid, which leads to a large number of triethylamine hydrochloride solid waste, there are irritating in the production process, solid waste and other shortcomings.
The acid-binding agent used in the synthesis of 2-(2-hydroxy-3-tert-butyl-5-methylbenzyl)-4-methyl-6-tert-butylphenyl acrylate (antioxidant 3052) is mostly triethylamine, but when triethylamine is used as the acid-binding agent, it not only reacts with acryloyl chloride to inactivate acryloyl chloride, but also has strong alkalinity, which will lead to further esterification of the product to produce acrylic acid diesters and other by-products. The results showed that the combination of pyridine, Na2CO3 and triethylamine as the mixed acid-binding agent greatly reduced the generation of by-products, and the selectivity of the raw materials was above 97%, and the yield was up to 80%. When the synthesized 3052 samples were added into the production of ABS resin, the chromatic aberration value of ΔE was less than 2.0, and reached the qualified standard of the industry. The color difference ΔE of the synthesized 3052 sample is less than 2.0, which meets the industry standard.
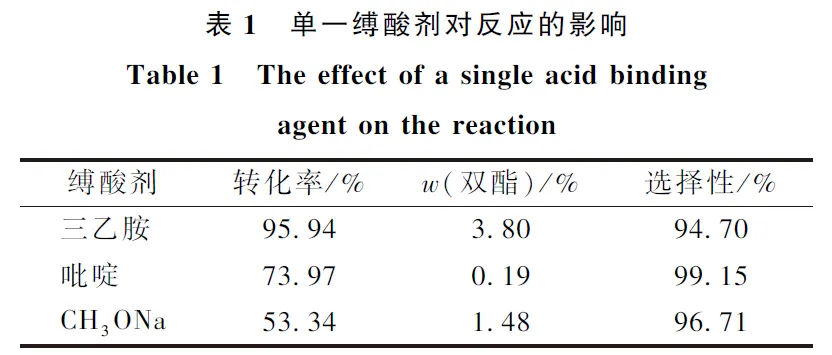

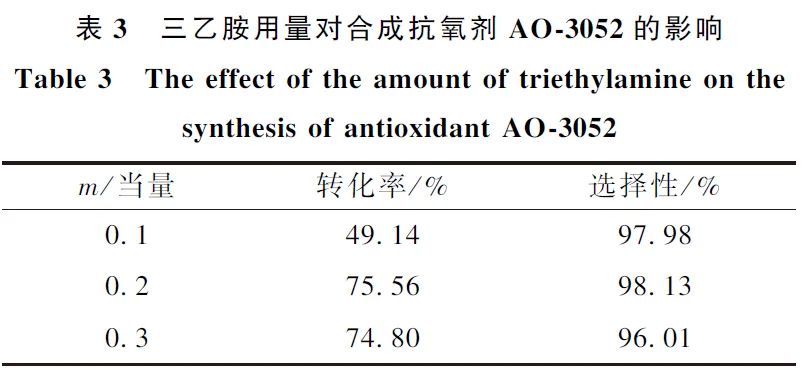

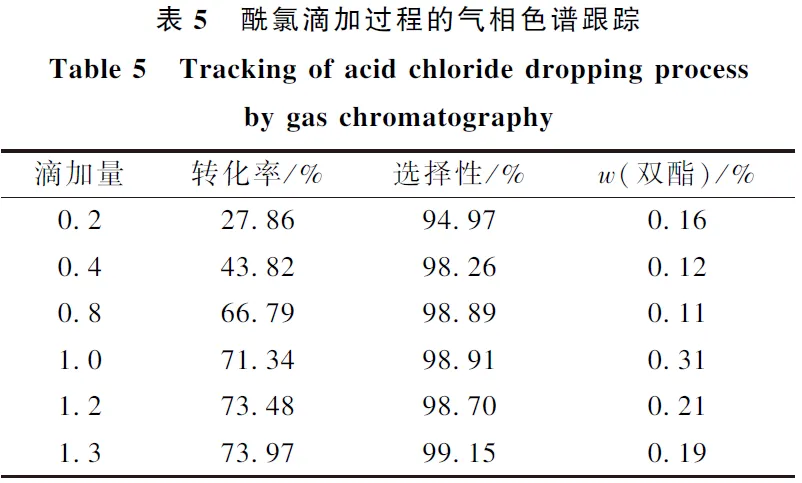
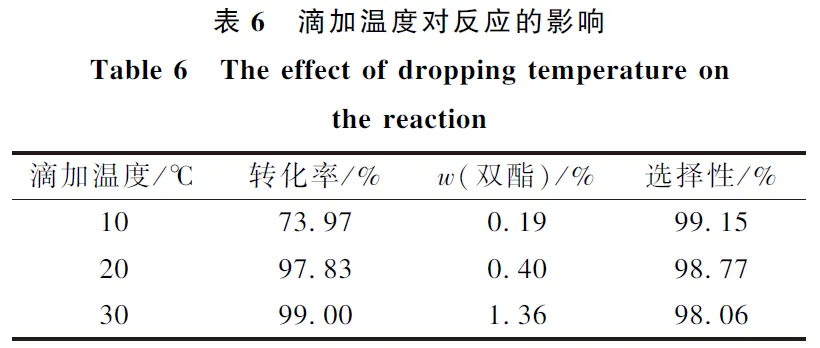
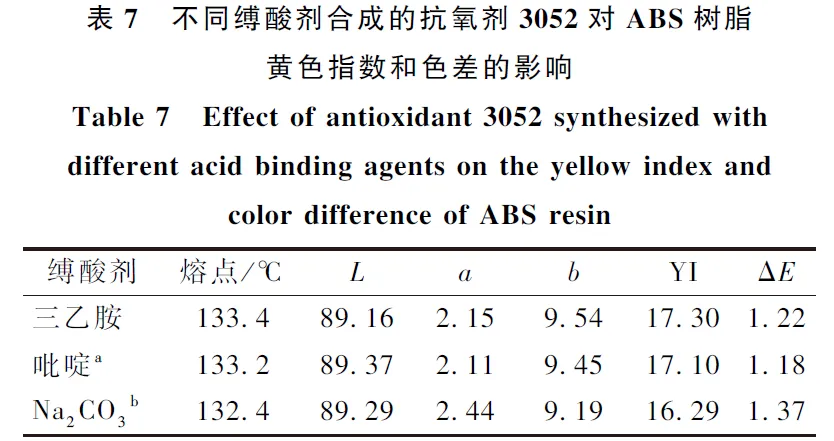
(1) Through the screening of different acid-binding agents, it was found that the inexpensive pyridine and Na2CO3 could partially replace triethylamine, which not only ensured the high conversion rate, but also the selectivity of the raw materials were above 97%.
(2)When the antioxidant 3052 generated by the partial substitution of pyridine and Na2CO3 for triethylamine was added into the production of ABS resin, the color difference ΔE was less than 2.0 in the industry standard, and reached the qualified standard.
Acid Scavenger (Acid Binding Agent) is often used to neutralize protons in a reaction system and reduce the effect of acid on the reaction. Commonly used acid binding agents are organic or inorganic bases, such as pyridine, triethylamine, DIEA, sodium carbonate, potassium carbonate, sodium acetate, and so on.
Picture 1: Some cations and anions that can be used to prepare basic ionic liquids
Taking the amidation reaction as an example, HCl is generated during the synthesis process, which has an inhibitory effect on amide synthesis. At the same time, HCl is prone to side reactions with raw materials, leading to the consumption of raw materials and even the decomposition of products, reducing the overall yield. Adding the corresponding acid-binding agent can neutralize HCl to generate salt, which is conducive to the positive direction of the reaction, while avoiding the impact of acid on the equipment and the environment and damage.
The introduction of acid-binding agent greatly improves the yield
It also causes high salt wastewater and back-end waste salt problem.
Acid binders do play a crucial role in increasing the reaction yield, but they also cause some subsequent problems. The use of organic or inorganic bases as acid binders results in the formation of a large number of by-products such as sodium, potassium or amine salts. Some inorganic salts formed after the reaction of acid binding are insoluble in the organic phase, forming the phenomenon of solid viscous material. The separation of products and waste salts is difficult, requiring a large number of organic solvents to participate in solid-liquid separation, so that a large number of waste solvents and waste salts are generated.
Adsorption separation: remove the organic matter in the liquid phase by adsorption, realize the by-production of waste salt.
The high salt wastewater of the process caused by the acid-binding agent contains various types of impurities and organic matter, and it is often difficult to realize the resource utilization of the by-product salt by directly adopting the evaporation and crystallization method. At the same time, contains a large number of organic matter high salt wastewater directly into the evaporation equipment, easy to cause high operating costs, equipment corrosion is serious, after evaporation of the material sticky coking and a series of operational problems.
We use resin adsorption process, for such process of high salt wastewater, first analyze the reaction mechanism, and then match the appropriate resin adsorption material, in order to achieve economic and efficient impurity enrichment and decolorization. The filtrate after adsorption then enters the conventional evaporation process, and the TOC content in the by-product salt obtained is greatly reduced.
Green acid-binding agent: choose acid-binding agent that is easy to separate, can be recycled and has less waste.
| Alkaline ionic liquid
Ionic liquid is a liquid composed entirely of ions and is a low-temperature molten substance, which is non-flammable and non-volatile, chemically stable, with low vapor pressure, and can be recycled. Alkaline ionic liquids can neutralize the acid in the reaction process and directly generate liquid-liquid systems, which makes the separation of products simple and easy, and does not produce solid hazardous waste.
In 2003, BASF (BASF) successfully developed the BASIL process to neutralize the HCl generated in the reaction by using ionic liquid as the acid-binding agent. after the reaction, the product and the ionic liquid are divided into two phases, which makes the product purification process very simple. The ionic liquid can be regenerated and reused after NaOH treatment. The current large-scale production and green synthesis of ionic liquids is still limited by technology and process.
Picture 2: Simple separation of products using ionic liquids as acid-binding agents
|Alkaline Ion Exchange Resin
Basic anion exchange resins are also used to try to act as acid binders in reactions. The resin material is extremely easy to separate from the reaction system and is easily regenerated without remaining in the product. In the case of ADC (diethylene glycol bicarbonyl dienophthalate) synthesis, for example, the solid basic ion exchange resin is used as an acid-binding agent, which prevents the raw material from being hydrolyzed due to the liquid alkali and consumes the raw material; at the same time, this method improves the yield and purity of the product, and stabilizes the product quality.
The research and application of ion exchange resin as acid-binding agent is still relatively small, the exchange capacity of alkaline functional groups, diffusion mass transfer in the reaction, etc. need more scientific data and industrialization experience.
The choice of acid binders is often centered on the yield of the product, followed by the combination of its alkalinity, stability, boiling point and other aspects. Green acid binders should be characterized by low toxicity, multiple recycling and easy separation to realize green production from the beginning of the reaction process.
Contact Us Now!
If you need Price, please fill in your contact information in the form below, we will usually contact you within 24 hours. You could also email me info@longchangchemical.com during working hours ( 8:30 am to 6:00 pm UTC+8 Mon.~Sat. ) or use the website live chat to get prompt reply.
| CHLUMIAO® 264 | CAS 128-37-0 | Antioxidant 264 / Butylated hydroxytoluene |
| CHLUMIAO® TNPP | CAS 26523-78-4 | Antioxidant TNPP |
| CHLUMIAO® TBHQ | CAS 1948-33-0 | Antioxidant TBHQ |
| CHLUMIAO® SEED | CAS 42774-15-2 | Antioxidant SEED |
| CHLUMIAO® PEPQ | CAS 119345-01-6 | Antioxidant PEPQ |
| CHLUMIAO® PEP-36 | CAS 80693-00-1 | Antioxidant PEP-36 |
| CHLUMIAO® MTBHQ | CAS 1948-33-0 | Antioxidant MTBHQ |
| CHLUMIAO® DSTP | CAS 693-36-7 | Antioxidant DSTP |
| CHLUMIAO® DSTDP | CAS 693-36-7 | Distearyl thiodipropionate |
| CHLUMIAO® DLTDP | CAS 123-28-4 | Dilauryl thiodipropionate |
| CHLUMIAO® DBHQ | CAS 88-58-4 | Antioxidant DBHQ |
| CHLUMIAO® 9228 | CAS 154862-43-8 | Irganox 9228 / Antioxidant 9228 |
| CHLUMIAO® 80 | CAS 90498-90-1 | Irganox 80 / Antioxidant 80 |
| CHLUMIAO® 702 | CAS 118-82-1 | Irganox 702 / Antioxidant 702 / Ethanox 702 |
| CHLUMIAO® 697 | CAS 70331-94-1 | Antioxidant 697 / Irganox 697 / Naugard XL-1 / Antioxidant 697 |
| CHLUMIAO® 626 | CAS 26741-53-7 | Ultranox 626 / Irgafos 126 |
| CHLUMIAO® 5057 | CAS 68411-46-1 | Irganox 5057 / Antioxidant 5057 / Omnistab AN 5057 |
| CHLUMIAO® 330 | CAS 1709-70-2 | Irganox 330 / Antioxidant 330 |
| CHLUMIAO® 3114 | CAS 27676-62-6 | Irganox 3114 / Antioxidant 3114 |
| CHLUMIAO® 3052 | CAS 61167-58-6 | IRGANOX 3052 / 4-methylphenyl Acrylate / Antioxidant 3052 |
| CHLUMIAO® 300 | CAS 96-69-5 | Irganox 300 / Antioxidant 300 |
| CHLUMIAO® 245 | CAS 36443-68-2 | Irganox 245 / Antioxidant 245 |
| CHLUMIAO® 2246 | CAS 119-47-1 | Irganox 2246 / BNX 2246 |
| CHLUMIAO® 1790 | CAS 40601-76-1 | Antioxidant 1790/ Cyanox 1790 / Irganox 1790 |
| CHLUMIAO® 1726 | CAS 110675-26-8 | Antioxidant 1726 / Irganox 1726 / Omnistab AN 1726 |
| CHLUMIAO® 168 | CAS 31570-04-4 | Irganox 168 / Antioxidant 168 |
| CHLUMIAO® 1520 | CAS 110553-27-0 | Irganox 1520 / Antioxidant 1520 |
| CHLUMIAO® 1425 | CAS 65140-91-2 | Irganox 1425 / Dragonox 1425 / Antioxidant 1425 / BNX 1425 |
| CHLUMIAO® 1330 | CAS 1709-70-2 | Irganox 1330 / Ethanox 330 |
| CHLUMIAO® 1222 | CAS 976-56-7 | Antioxidant 1222 / Irganox 1222 |
| CHLUMIAO® 1135 | CAS 125643-61-0 | Irganox 1135 / Antioxidant 1135 |
| CHLUMIAO® 1098 | CAS 23128-74-7 | Irganox 1098 / Antioxidant 1098 |
| CHLUMIAO® 1076 | CAS 2082-79-3 | Irganox 1076 / Antioxidant 1076 |
| CHLUMIAO® 1035 | CAS 41484-35-9 | Irganox 1035 / Antioxidant 1035 |
| CHLUMIAO® 1024 | CAS 32687-78-8 | Irganox 1024 / Antioxidant 1024 |
| CHLUMIAO® 1010 | CAS 6683-19-8 | Irganox 1010 / Antioxidant 1010 |
