Data introduction of commonly used UV monomers in UV inkjet
There are many kinds of UV light curing monomers, and the classification method is also complicated. For example, according to the mechanism of the curing reaction, they can be divided into free radical curing type and cation curing type; according to the number of functional groups involved in the reaction, they can be divided into monofunctional, bifunctional, trifunctional and Multifunctional monomer. This article intends to use the following diagrams to classify, hoping to give some inspiration or help to friends who are engaged in UV inkjet formulation design.
At present, acrylates and some nitrogen-containing monomers are widely used in UV-curable inkjets. Therefore, this article also focuses on summarizing these monomers.
Friends who are familiar with inkjet formulation design know that monomer odor, dilution and Tg point are more important data.
What factors are usually related to the odor of monomers (Odor) The molecular weight of the acrylate is small, but it has almost no smell. If you think about it carefully, you will find that hydrogen bonds are formed between the molecules, which makes it difficult to escape, so we are thinking about whether a monomer has a smell When it is large, it should be judged from the data of the vapor pressure of the substance. The higher the vapor pressure, the easier the substance is to emit, so the concentration of the substance we can receive is relatively high. Once the dose is up, the taste will come out easily. Of course, there are other factors, such as a substance that is easy to decompose at room temperature. The odorous small molecular components that escape will also have odor. Compounds containing sulfhydryl groups that are well known in the light curing industry should belong to this category. In addition, some odors come from the reactions added in the production process of a certain material. Therefore, some monomer suppliers will provide two or several versions of monomers, most typically Toluene free or High purity monomers.
The viscosity of the monomer (Viscous), we usually think that the lower the viscosity of the monomer, the better the dilution performance. In the acrylate system, most monomers conform to such a rule. Viscosity reflects the strength of the intermolecular force. At the same temperature, the stronger the intermolecular force, the higher the viscosity. The dilution force is not only related to the viscosity of the monomer, but also related to the polarity of the monomer. The viscosity of acrylates such as medium-length carbon chains (C8~C10) is not high, but it is sometimes used in formulations. In addition to affecting the curing speed and the hardness of the film layer, the biggest hidden danger is that the polarity of the carbon chain part is relatively small, and the intermolecular force between the monomer and itself is small, so it may also interact with the resin molecules. The force is also small, and it is likely that the resin cannot be dissolved well after adding more, that is, the compatibility with the ink system may be problematic.
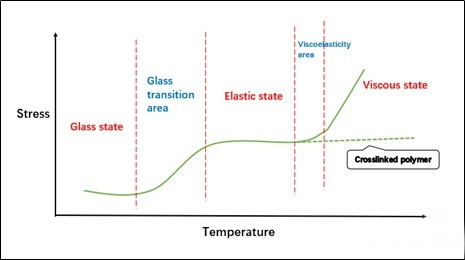
Tg is the glass transition temperature. The meaning of this parameter is understood according to polymer physics. It is the critical value of the temperature when the chain links in the polymer can just rotate freely. It is different from the melting point and boiling point of small molecules. It is not a The sudden change of endothermic and exothermic temperature is a wide range of temperature changes. In order to facilitate the characterization, the value is usually taken at a certain point in this range. Of course, the characterization data has a great relationship with the measurement method, so the same sample in different The data obtained under the test conditions may vary greatly, so the Tg data introduced in the following article is only a reference. Now that we understand the meaning of Tg, we can also have a little deeper understanding of a more important concept of “three states and two transitions” in polymer materials (strictly speaking, this is for amorphous polymers).
The understanding of the glass state is similar to the glass we have seen. The glass is actually uncrystallized, but at room temperature, it is a “solid” system, but strictly speaking it should be “liquid”, only the temperature conditions It only restricts some movement of its molecules. For an amorphous polymer, when it is in a glass state, the “segment” cannot move, and it is in the so-called locked state. At this time, the system is as hard as glass.
The high elastic state is actually a state in which the “chain segment” can move freely but the entire polymer chain cannot slide yet. At this time, the system has good elasticity.
The viscous flow state is the state in which the polymer chain can move, similar to our common liquid. Of course, for the cross-linked polymer, the stress will not increase when it reaches the viscous flow temperature, after all, the movement of the polymer chain is constrained.
“Two transitions” is the connection between “state” and “state”, mainly including glass state and viscoelastic state. It is not difficult to understand that when the glass state is in a lower temperature range, that is, when the Tg point we are talking about is very low, it may be in a viscoelastic state at room temperature. “Can’t get through”. Therefore, when designing the formula of hard ink, we hope that the Tg point is high, which will “dry thoroughly” and the surface hardness is also high; when designing the formula of soft ink, it is slightly more difficult, and we hope that the Tg of the cured film layer can be in the elastic region. , so that it can have better tensile properties.
Refractive Index, usually the larger the refractive index, the better the gloss, because the light is easier to refract back and forth on the superficial surface. In addition, the high refractive index can also help to improve the coverage of the pigment, but if it is not too high , this positive effect is almost negligible.
We will mainly discuss these parameters later.
2. UV inkjet commonly used monofunctional acrylate monomer
2.1 Monofunctional acrylates with saturated carbon chains of different lengths
The structure diagram and the structure and data table of partially pure saturated carbon chain acrylate are as follows:
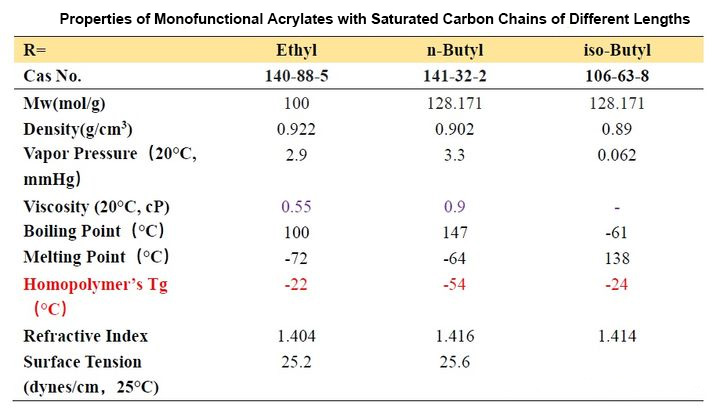
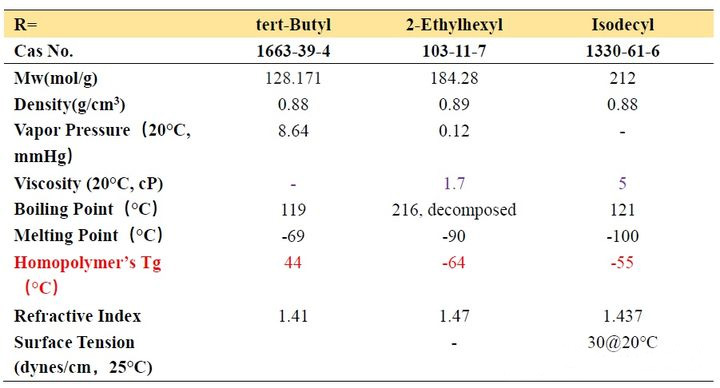

From Table 2-1, we can observe that the Tg of homopolymers with different carbon chain lengths varies greatly. As the number of carbon atoms increases, the Tg value first decreases and then increases. When the number of carbon atoms increases to a certain value, it constitutes a longest “chain segment”, and continuing to increase is equivalent to the next “chain segment”, so the Tg changes accordingly. Let’s look at several acrylates with isomers of n-butyl, iso-butyl and tert-butyl. We find that n-butyl has the lowest Tg point and tert-butyl has the highest, which means that the steric hindrance has the opposite effect. The movement of the “chain segment” has a greater influence.
In addition, we look at the Tg of the two decyl ester isomers. It can be seen that the length of a “chain segment” is about C5. If the value is larger, the Tg point is higher.
How do we apply this acrylate with different carbon chains to the ink?
First of all, we examine the vapor pressure data. Ethyl and butyl esters with smaller molecular weights usually have a strong odor and are rarely used in ink formulations. Although decyl esters and lauryl esters with slightly longer carbon chains have lower viscosity and lower Tg, It seems that it can be applied to soft ink to get very soft and rubbing resistance, but we should not ignore their polarity. Such monomers should not be added too much. Generally, they are used as additional monomers for internal plasticization. Monomers are widely used in the synthesis of adhesive resins, and the addition amount does not need to be too much. One is the compatibility just mentioned, and the other is that when the addition amount is large, the curing will be slow. These monomers are also used in 3D inkjet printing, and because of their compatibility, they give printed models a waxy look and feel.
Among the above-mentioned monomers, BA, 2-EHA, ISODA, 2-PHA, LA and SA are all available from major manufacturers of photocurable raw materials.
2.2 Monofunctional acrylates with non-aromatic carbocycles
In addition to the alcoholic hydroxyl group, other parts of the alcohol before acrylated have a ring formed by carbon atoms. Monomers with carbocyclic rings are also used in the field of inkjet, but the amount is relatively small. Below we discuss its possible applications in terms of its structural parameters.
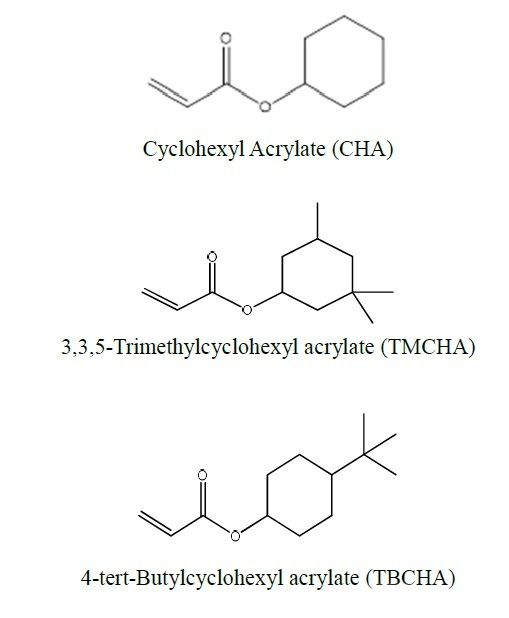
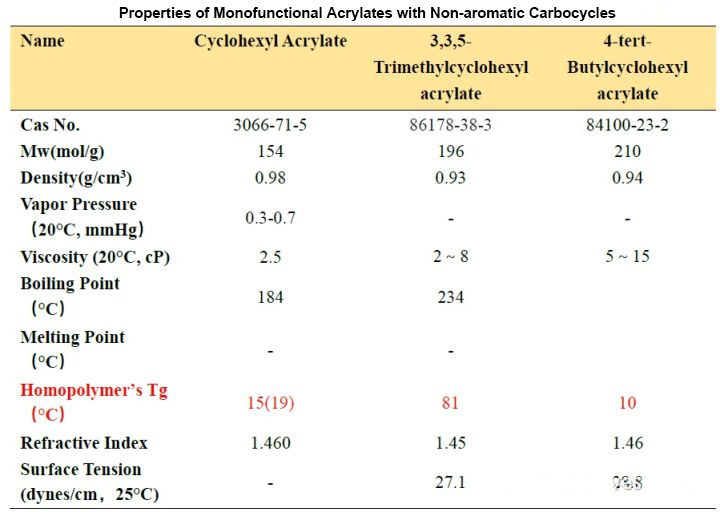
Comparing these three side by side is because only the substituents at different positions on the cyclohexyl group are different, and the effect on the Tg of the cyclohexyl group is so different. The difference between TMCHA and TBCHA and CHA can be several tens of degrees Celsius. It can be seen that The more rigid substituents like methyl groups are directly substituted on the ring, the more “harder” the polymer will be after curing. In fact, the saturated ring structure also has the advantage of water resistance and better yellowing. The water resistance is good because the polarity of the groups is low. According to the experience of “similar compatibility”, the hydrophobic effect is obvious. The good yellowing resistance is because it is not like monomers containing an aromatic ring structure, which is easily oxidized by complex factors such as light and heat in the environment at the methyl group substituted by the aromatic ring to form a “quinone” structure. Conjugation leads to a red shift of its absorption wavelength, and there is a certain wavelength absorption in the visible light region, so that the result of yellowing can be seen macroscopically.

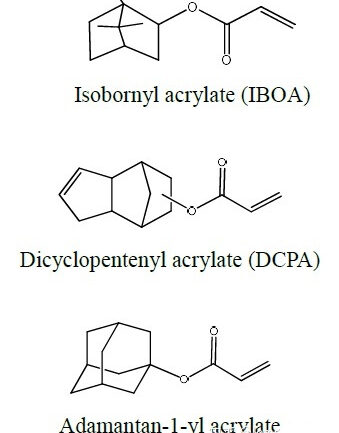
Next we look at a group of acrylates with bi- or polycyclic structures. Without exception, these acrylates have a higher Tg and a relatively faster cure rate.
The most well-known is isobornyl acrylate (IBOA), which has a special odor, similar to camphor oil. The synthesis of this type of monomer is usually obtained from the corresponding olefin through Lewis acid catalysis to obtain hydroxyl group and carry out with acrylic acid. Esterification, process and cost considerations prevent the purity of such products from being very high. In addition, there are dicyclopentadiene (DCPA), adamantane (Adamantane) series of acrylates with rings, but the price is not cheap, the corresponding data is difficult to collect comprehensively, it should be applied in special fields. Only the structure and some parameters are listed for reference.

If you need COA, MSDS or TDS of UV monomers, You could email me info@longchangchemical.com during working hours ( 8:30 am to 6:00 pm UTC+8 Mon.~Sat. ) or use the website live chat to get prompt reply.
You can click on the next article to read and understand What is UV Ink? What is the difference between uv ink and ordinary ink?