【English name】Ethyl Vinyl Ether
【Molecular formula】 C4H8O
【Molecular Weight】72.11
【CAS Registration Number】[109-92-2]
【Structural formula】CH3CH2OCH=CH2
[Physical properties] bp 33 oC, d 0.753 g/cm3. It is soluble in most organic solvents and can be used in a variety of organic solvents.
[Preparation and Commodity] This reagent is sold in chemical reagent companies at home and abroad.
[Precautions] This reagent is a highly volatile and flammable chemical. It is recommended to store it in a low-temperature and dry place and use it in a fume hood.
Ethyl vinyl ether is mainly used as a protective group of hydroxyl group, vinyl transfer reagent, and participates in cyclization addition reaction in organic synthesis.
Among the many protecting groups for hydroxy groups, the α-ethoxy ethyl ether (EE) formed by ethyl vinyl ether and hydroxy is one of the most commonly used and convenient protecting groups. The reaction generally requires a strong acid catalyst, TFA and TsOH can be used for this purpose, the most commonly used is PPTS. The reaction is usually completed by stirring for several hours at room temperature. In most cases, the yield of the product is above 95% (Formula 1) [1,2]. The EE protecting group can easily complete the deprotection reaction under acidic conditions. PPTS-EtOH and aq. HCl-MeOH are recommended methods (Equation 2)

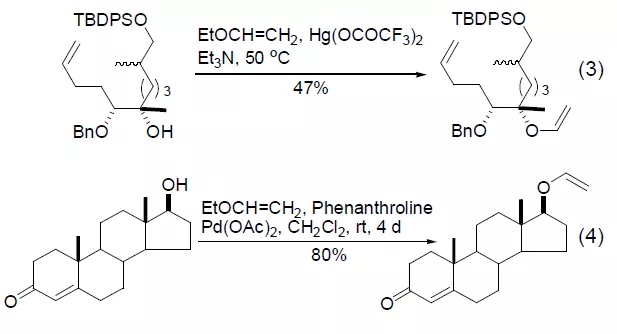
Under the action of the mercuric acetate derivative catalyst, ethyl vinyl ether and hydroxyl undergo a vinyl transfer reaction to generate the corresponding vinyl ether product (Formula 3) [5]. If a metal palladium catalyst is used, it can effectively avoid the use of a metal mercury catalyst (Equation 4) [6]. This reaction is more meaningful because the product can undergo further condensation reactions or olefin metathesis [7,8].
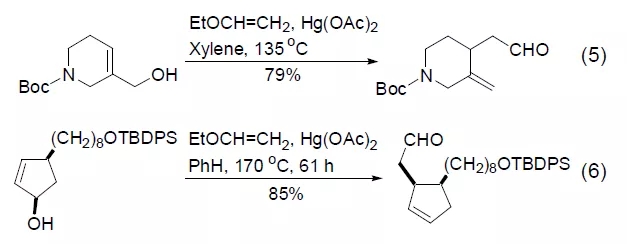
Ethyl vinyl ether and allyl hydroxyl undergo a vinyl transfer reaction to generate the corresponding vinyl ether product. The reaction is usually completed under the catalysis of mercuric acetate. Heating the product in toluene or xylene can induce the corresponding Claisen rearrangement, making the reaction of important synthetic value. Under normal circumstances, the two-step reaction process can be completed under the conditions of “one-pot cooking” (Equation 5, Equation 6) [9~11].
In the presence of Lewis acid catalysts, ethyl vinyl ether can also undergo the corresponding cyclization reaction [12,13].
