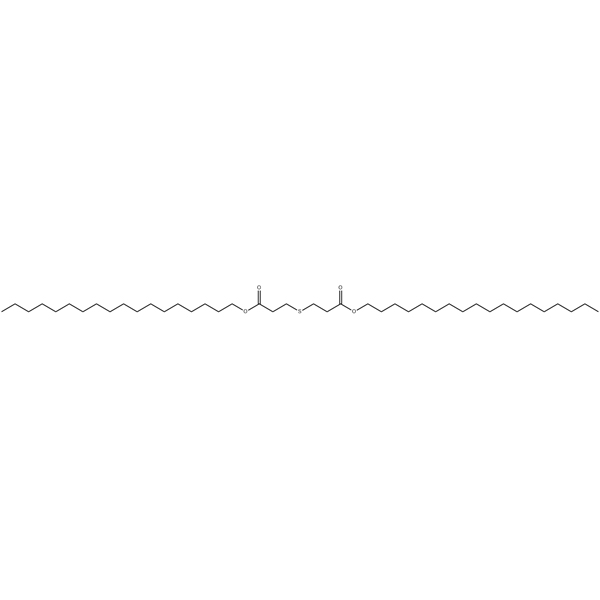
抗氧化剂 DSTDP 应用场景
1.塑料工业
提供持久的热氧化稳定性,防止在加工和使用过程中出现降解、褪色和机械性能下降。
聚烯烃(如聚丙烯 PP、聚乙烯 PE)、苯乙烯聚合物(如 ABS、PS)、
其他树脂
2.橡胶工业
作为一种无污染的辅助抗氧化剂,可防止橡胶制品因热氧化老化而变硬、变脆或开裂。
各种合成橡胶和产品。
3.机油和润滑油
抑制机油、润滑油和润滑脂的氧化酸败,延长使用寿命并保持性能稳定性。
工业润滑油、润滑脂和肥皂。
4.化妆品和个人护理
作为一种抗氧化剂和紫外线稳定剂,它能保护产品成分,帮助皮肤抵御自由基和紫外线的伤害。
面霜、乳液和其他护肤品。
💡 核心作用机制和使用特点
要了解 DSTDP 的应用,就必须了解它的两个核心特征:
作为一种二级抗氧化剂: 它不会直接捕捉自由基,而是高效分解氢过氧化物,将其转化为稳定的醇类,从而中断氧化链反应。这一功能与能够捕捉自由基的酚类初级抗氧化剂(如 Revonox® 501)形成了完美的互补。
协同效应: 当 DSTDP 与酚类初级抗氧化剂结合使用时,二者会产生 "1+1>2 "的协同效应,显著提高材料的整体抗氧化能力。这也是其在配方中得到广泛应用的一个重要原因。


夏洛特韦斯特 -
优质的服务、快速的回复、无忧的物流、愉快的购物!