Domestic research progress of hindered amine light stabilizers
In recent years, in order to further improve the light stability performance of hindered amine light stabilizers and broaden their application fields, the research on hindered amine light stabilizers is still very active, with high molecular weight, low alkalinity, reactive and multifunctionalization. The current research leadership strives for better performance, better functions, lower cost, and wider application range without losing its light stability performance. This article will briefly discuss the research on the improvement direction of various light stabilizers, paying particular attention to some domestic research progress.
Since hindered amine light stabilizers are often used in high specific surface area polymer materials, the biggest disadvantage of low molecular weight hindered amine light stabilizers is poor extraction resistance, volatile, and easy to lose during use.
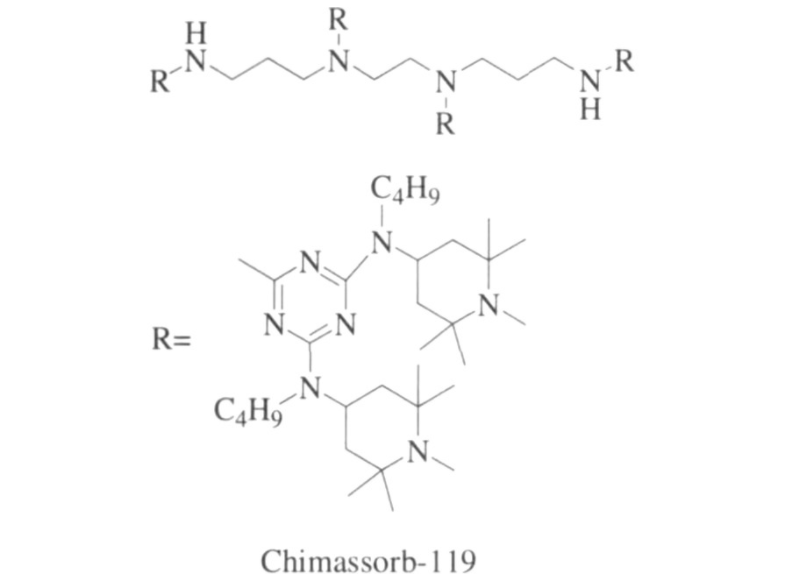
In order to solve this problem, Ciba-Geigy launched the monomeric polymer hindered amine light stabilizer Chimassorb 119. This light stabilizer has good light stability and is widely used in polyolefins and polystyrenes. In polymer materials, the structure is shown in Figure 1.
Studies have shown that although hindered amine light stabilizers with excessively high molecular weight are resistant to extraction and difficult to volatilize, they will reduce their migration properties in polymer materials and affect the normal performance of their light stabilizing effects. The way to solve this contradiction is to seek an optimal molecular weight range, usually controlled at 2 000 to 3 000 g/mol to achieve a more balanced use effect.
Since the high alkalinity of most hindered amine light stabilizers made it impossible to be used in acidic environments and was difficult to compound with acidic additives, people began to pay attention to hindered amine light stabilizers with low alkalinity in the late 1980s. Sexualized research. Ciba-Geigy developed the product Tinuvin 123 through N-alkylation reaction, which can be used in combination with halogen-containing flame retardants and sulfur-based auxiliary antioxidants, and also has excellent light stabilization effects. At present, the low-alkalinity products of hindered amine light stabilizers include Chimassorb 119, Tinuvin 371, Tinuvin 152 of Ciba-Geigy and Cyasorb UV 3529 of Cytec.
On the basis of the development of dendritic light stabilizers, Chen Wei from Tianjin University and others designed and synthesized the low-basic hindered amine light stabilizers shown in Figure 2 based on diamine compounds, and carried out systematic Light stability test.
The class I dendritic hindered amine light stabilizer they designed and synthesized has a pH of 9.6 to 9.7, which is strongly alkaline and can be used in a relatively alkaline environment. In addition, the melting point of this kind of light stabilizer is 50-80 ℃, which is suitable for the processing of most materials, and its light transmittance is good. The pH of the class II stabilizer after N-methylation is reduced to 8.3~8.4, which can be used in a relatively alkaline environment, and has excellent light transmittance, especially Ⅱd and Ⅱe are more prominent, and can even be compared with Commercially available hindered amine light stabilizers Tinuvin 770, Tinuvin 622 and Chimassorb 944 are comparable.
Ia, Id, IIe were selected for application performance testing, including oxidation induction period experiment, artificial climate accelerated aging experiment, carbon arc lamp artificial climate aging experiment, xenon lamp artificial climate aging experiment, fluorescent ultraviolet lamp artificial climate aging experiment, and various results The performance is excellent. The antioxidant effect of Ia and IIe is greater than that of the antioxidant Irganox B215, and the mechanical properties of the material can be better maintained, and the advantages of light stability are very obvious.
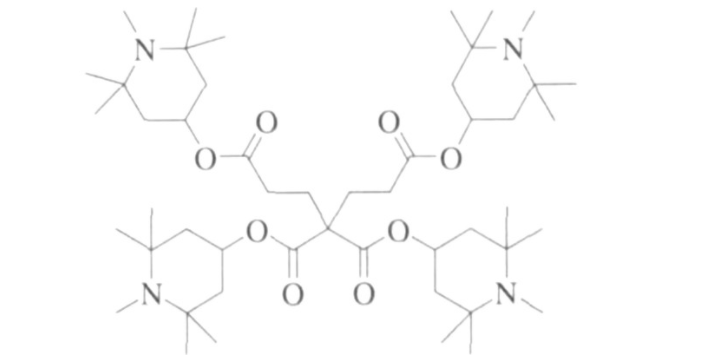
On this basis, Beijing Tiangang Auxiliary Co., Ltd. and Tianjin University have jointly developed a lower alkaline dendritic hindered amine light stabilizer based on dicarboxylic acid esters. The structural formula is shown in Figure 3. However, although low alkalinity broadens the application range of hindered amine light stabilizers, it also brings other problems.
Take Tinuvin 770 as an example. From a mechanism point of view, nitroxide radicals trap free radicals in polymer materials to generate an amine ether structure similar to Tinuvin 123, that is, Tinuvin 123 loses the light stability of the original hindered amine light stabilizer twice. ability. Based on the above reasons, the alkalinity of hindered amine light stabilizers should be selected according to the actual working environment. Under non-acidic conditions, it is not necessary to pursue low alkalinity too much. The light stabilizing effect of hindered amine light stabilizers with high alkalinity is not Stabilizers that are not inferior to low alkalinity, such as Chimassorb 944 are 1000 times more alkaline than Tinuvin 622, and the light stabilizing effect is also much higher.
The introduction of other groups into the hindered amine light stabilizer molecule to impart other functions to the hindered amine light stabilizer is an important trend in current research. For example, the introduction of hindered phenol groups into hindered amine light stabilizer molecules can exert the heat and oxygen aging resistance of light stabilizers. Tinuvin 144 and Sanol LS 2626 are successful examples.
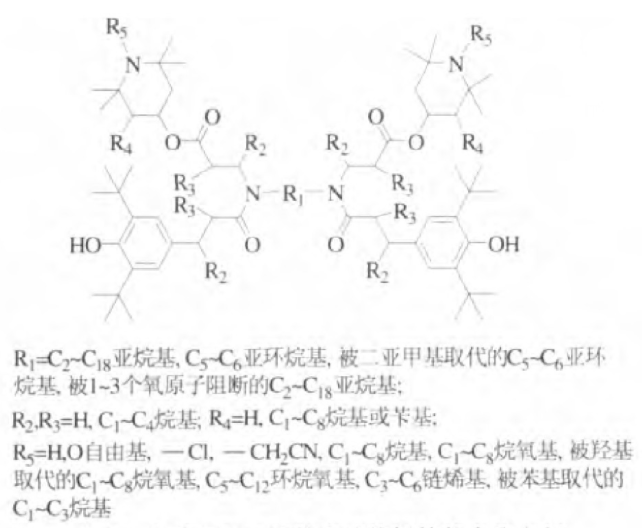
Beijing Tiangang Auxiliary Co., Ltd. and Tianjin University have designed and synthesized a series of multifunctional light stabilizers containing hindered phenols. Studies have shown that these hindered phenolic light stabilizers have good extraction resistance and thermal oxidation resistance. The compatibility of polymer materials is relatively high, and its structural formula is shown in Figure 4.
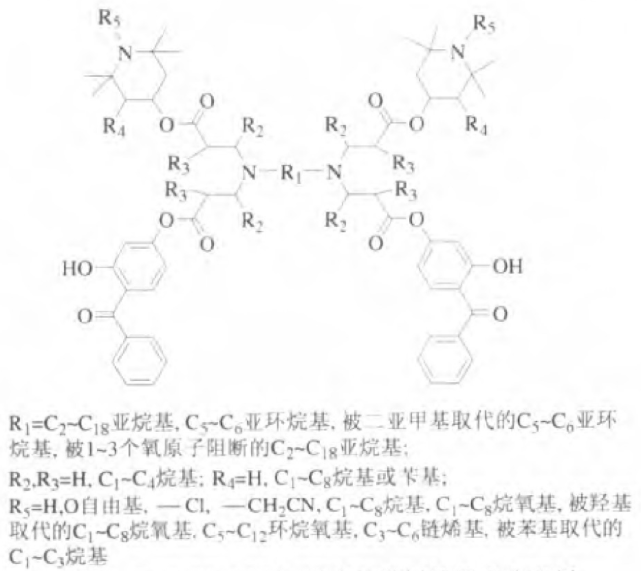
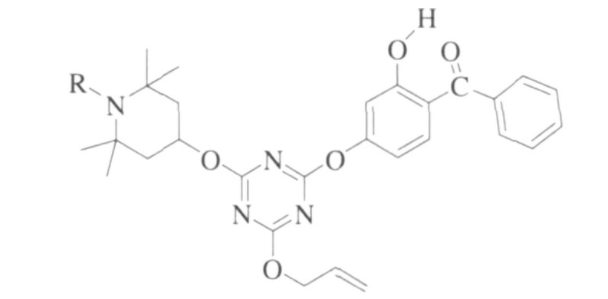
In addition, the introduction of benzophenone or triazine structure into the molecular structure can make it have the function of absorbing ultraviolet light. Jezy Zakrazewski and others have done a lot of work to synthesize many hindered amine light stabilizers with ultraviolet absorbing function. Beijing Tiangang Auxiliary Co., Ltd. designed and synthesized hindered amine light stabilizers containing benzophenone groups based on diamine compounds. The application performance test showed that it has excellent ultraviolet absorption function and anti-photooxidative aging function. Its structural formula is shown in Figure 5.
Shanxi Chemical Industry Research Institute organically combined hindered amines and phosphites to develop GW-540. T Konstantinova, Zuo Hongliang and others combined hindered amines with benzotriazole to prepare hindered amine light stabilizers. Both show a good light stabilization effect and have the characteristics of functional diversity.
If a reactive group is introduced into the hindered amine molecular structure, it can be bonded to the polymer backbone during the polymer preparation process to form a polymer material with permanent light stabilization effect, which can overcome the hindered amine light stability The loss of the agent due to physical migration or volatilization. In recent years, reactive hindered amine light stabilizers have developed rapidly, and products have emerged one after another. For example, Luchem HAR100 launched by Elf Atochem has a reactive oxalyl hydrazide group in its molecule, which can be combined with amino, isocyanate and epoxy groups. The group reacts to bond to the backbone of various polymers.
The research team of the Bulgarian University of Metallurgy and Chemical Technology is at the leading level in the research of reactive hindered amine light stabilizers. They synthesized piperidinol, allyl alcohol, benzophenone and benzotriazole compounds in the presence of phase transfer catalysts. The structural formula shown in Figure 6 is a reactive hindered amine light stabilizer, in which piperidinol is the functional group of the hindered amine light stabilizer to prevent photooxidation on the surface of the material, and benzophenone or benzotriazole is ultraviolet The absorber can prevent the deep layer of the material from being degraded by light, and the acrylic base provides the ability of the stabilizer to bond with the material, making it a part of the polymer material.
Shanxi Provincial Research Institute of Chemical Industry has also conducted in-depth research in the field of reactive hindered amine light stabilizers. The GW-628 currently being promoted has excellent performance and ultraviolet absorption function. Its biggest advantage is that it successfully solves the problem of reactive hindered amine light stabilizers. The problem of difficult grafting of stabilizers. Studies have shown that, without changing the processing methods of the products, the light stability of GW-628 to agricultural film and injection molded polyene products is better than Chimassorb 994.
Hindered amine light stabilizers are a class of light stabilizers with excellent performance. They are still the focus of research and development. With the continuous emergence of new products, their application scope is also expanding. In the future, they will replace traditional light stabilizers. Become the leading product in the light stabilizer industry.
In recent years, foreign research and development of new varieties of hindered amine light stabilizers has been relatively active, especially in the field of multifunctional and reactive light stabilizers, while the domestic is relatively backward. To this end, researchers need to focus on strengthening basic research, increase technical research on key intermediates, and selectively focus on the development of new varieties with independent intellectual property rights. I believe that through the unremitting efforts of generations of scientific researchers, the research on hindered amine light stabilizers in China will surely embark on a path of rapid development.
Same series products
Contact Us Now!
If you need COA, MSDS or TDS, please fill in your contact information in the form below, we will usually contact you within 24 hours. You could also email me info@longchangchemical.com during working hours ( 8:30 am to 6:00 pm UTC+8 Mon.~Sat. ) or use the website live chat to get prompt reply.
This article was written by Longchang Chemical R&D Department. If you need to copy and reprint, please indicate the source.


Astra Hilarius Elana
Excellent post. I absolutely love this website. Keep writing!
Carita Jefferey Hitoshi
Thanks for the marvelous posting! I quite enjoyed reading it, you are a great author. I will be sure to bookmark your blog and will often come back sometime soon. I want to encourage that you continue your great posts, have a nice morning!