In the beginning stage of oxidation, as the temperature rises it will intensify the thermal degradation reaction, the oxidative degradation reaction of PC materials is an autocatalytic process, the carbonyl and hydroxyl groups in the polymer may affect the neighbouring chemical bonds, causing them to split into free radicals, and these free radicals can react with oxygen to generate new free radicals, and so on, week by week, repeatedly cycling the oxidative reaction according to the free-radical chain course has been carried out, with the formation of peroxides and other oxygen-containing groups on the polymer chain will happen to break the macromolecular chain. With the formation of peroxide and other oxygen-containing groups on the polymer chain, the breakage of the polymer chain will occur, and in the chain termination stage, the combination of free radicals will cause cross-linking of polymers, no matter whether the chain breakage or cross-linking will cause irreversible changes in the mechanical properties of the material, and the formation and accumulation of a variety of carbonyl compounds will cause the material’s discolouration, affecting its appearance.
Therefore, the thermal stability of PC can be improved by choosing to add suitable antioxidants to prevent or attenuate the discolouration of PC caused by thermal degradation. In this case, the peroxide decomposer reduces the number of reactive free radicals that need to be terminated by the chain-terminating antioxidant; and the chain-terminating antioxidant likewise reduces the burden of the peroxide decomposer. The -OH contained in the hindered phenolic antioxidant competes with the polymer for the peroxyl radicals formed in auto-oxidation, and through the transfer of hydrogen atoms, a stable antioxidant radical is formed, which, in turn, has the ability to capture reactive radicals and inhibit the polymer oxidation process. Therefore, by adding antioxidants, the thermal stability of PC can be improved, thus improving the colour of PC products.
The effect of different antioxidants on product colour
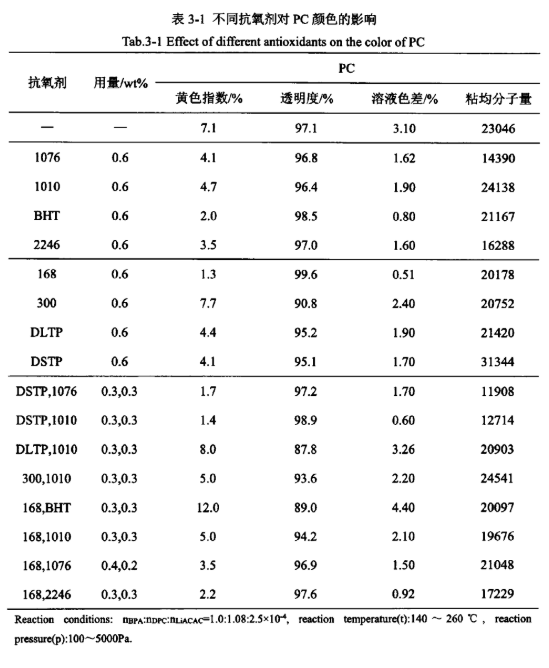
Antioxidants can be added during PC synthesis to attenuate or prevent oxidative degradation side reactions from occurring, thus maintaining the appearance of the PC product colour. The effect of antioxidants on product colour in PC synthesis was examined, and the results are listed in Table 3-1. From Table 3-1, it can be seen that without adding any antioxidant, the yellow index and solution colour difference of the PC products produced were high. When adding the main antioxidant 1076, 1010, 2246, BHT, auxiliary antioxidant 168, DLTP, DSTP, can significantly reduce the yellow index of PC products and solution colour difference, of which antioxidant 168, BHT can also improve the transparency of the product, antioxidant 1076, 1010, 2246 basically has no effect on the transparency of the antioxidant DLTP, DSTP slightly Decrease the transparency of the product, and add auxiliary antioxidant 300 effect is not good, increased the yellow index of PC products and solution colour difference, and also reduces the transparency of the product. Among the above antioxidants, only 1076 and 2246 significantly affected the viscosity average molecular weight of the products, and the addition of other antioxidants had less effect on the viscosity average molecular weight of the products. Comparing the product’s yellow index, transparency, solution colour difference and MOA, the order of the effects of the main and auxiliary antioxidants is 168>BHT>2246>DSTP>1076>DLTP>1010>300 in the following order.
Antioxidant DSTP and 1076, DSTP and 1010, 168 and 1076, 168 and 2246 of the compounding effect is better, can varying degrees to reduce the product’s yellow index and solution colour difference, improve its transparency; antioxidant 300 and 1010, 168 and 1010 of the compounding effect is not very obvious, although it can be a certain degree of reduction in the product’s yellow index and solution colour difference, but The effect of compounding antioxidant 300 with 1010 and 168 with 1010 is not very obvious, although it can reduce the yellow index and solution colour difference to some extent, but it reduces the transparency of the product; while the effect of compounding antioxidant DLTP with 1010 and 168 with BHT is not good, which increases the yellow index and solution colour difference of the product and reduces its transparency. Among them, only the antioxidant DSTP and 1076, DSTP and 1010 compounding addition, the viscosity average molecular weight of the product has a greater impact, and the other antioxidants compounding addition, the viscosity average molecular weight of the product has a smaller impact. Comparing the yellow index, transparency, solution colour difference and viscosity average molecular weight of the products, the order of the superiority and inferiority of the effects of the main and auxiliary antioxidants after compounding and adding are:
168+2246>DSTP+1010>DSTP+1076>168+1076>168+1010>300+1010>DLTP+1010>168+BHT.
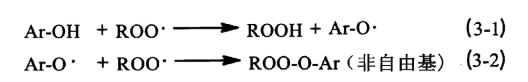
The above main antioxidants belong to the hindered phenolic antioxidants, whose function is to capture the free radicals [see formula (3-1), formula (3-2)] and generate stable non-free radicals ROO-O-Ar, so that they no longer participate in the oxidation cycle. The key to the antioxidant effect lies in the reactive hydroxyl group it contains, and the reactivity of the hydroxyl group with free radicals is affected by the spatial site resistance of its neighbouring R group and the electronic effect of the opposite R group. the larger the R group is, the larger the site resistance is, and the smaller the reactivity is. when the R group is an electron-donating group (e.g., methyl, tertiary-butyl), it accelerates the separation of the hydrogen atoms and the oxygen atoms on the hydroxyl group, which in turn improves the rate constant of the hydroxyl group’s reaction with free radicals kinh, reducing the phenolic radical’s pro-electronic substitution group constant, i.e., increasing the radical capture number n, thus increasing the antioxidant activity; when the R group is an electron-withdrawing group (e.g., nitro or hydroxyl), it reduces the antioxidant activity of the phenolic antioxidant. The above phenolic antioxidants to BHT’s effect is the best, because of its molecular structure in the neighbouring R group is tert-butyl, its spatial site resistance is small, the R group is also an electron-donating group methyl, have increased its antioxidant activity.
During the PC oxidative radical chain reaction, the production and accumulation of hydroperoxides is the most critical step in the degradation of PC materials, and when a certain concentration of hydroperoxides is generated, the autoxidation counter of the radical branched chain advances rapidly. Therefore, it is necessary to add auxiliary antioxidants to decompose hydroperoxides during PC synthesis. Phosphite antioxidant 168, thioester antioxidant DLTP and DSTP are very effective decomposers of hydroperoxides, which can make the very unstable macromolecule hydroperoxides to generate stable and inactive products and terminate the chain reaction. Among them, the phosphite antioxidant 168 is the most effective. Because the antioxidant 168 in addition to a good decomposition of hydroperoxides, but also has a good ability to protect the colour. The main component of antioxidant 168 is phosphite, through the Arbuzov reaction [see formula (3-3), (3-4)] can capture the residual harmful chloride ions in the reaction material system, the formation of stable compounds <(RO)3PR> + CI-, preventing the initial discolouration of PC macromolecules.
At the same time, the phosphorus atom in the molecular formula of antioxidant 168 contains two lone pairs of electrons, which is a good chelating agent, which can react with the residual harmful metal ions in the system, such as Fe2+, Mn2+, etc. [see formula (3-5)] to form a chelate, avoiding the reaction of the non-ferrous metal ions and phenolic hydroxyl group in the molecule of the PC to form the dark-coloured compounds, which will guarantee the appearance of the PC colour and improve the transparency of the product.
According to the literature, the main and auxiliary antioxidants can play a good antioxidant synergistic effect when they are added to polymer materials together. In the antioxidant process, the hindered phenolic antioxidant captures PC oxidation radicals, and the auxiliary antioxidant decomposes hydroperoxides, and the two kinds of antioxidants are added in a certain ratio of compounding, which theoretically can get the antioxidant system with better performance than any single component. However, due to the difference in molecular structure between the antioxidants, as well as the reaction’s own characteristics, resulting in different main and auxiliary antioxidant compounding effect of the difference.
The effect of antioxidant dosage on PC colour
Antioxidants can slow down the thermo-oxidative degradation reaction in the process of synthesising PC, improve its stability and reduce the degree of yellowing of PC. Therefore, the dosage of antioxidant also has a certain effect on the appearance of PC colour. The effect of different dosage of antioxidant 168 on PC colour is shown in Figure 3-2.
As can be seen from Figure 3-2, the dosage of antioxidant 168 has a very obvious effect on the PC yellow index, transparency and solution colour difference. With the increase of the dosage of antioxidant 168, the appearance quality of PC products improved significantly, when the dosage is 0.6wt%, the appearance quality of PC products is better, the yellow index is only 1.3%, the transparency reaches 99.6%, the solution colour difference is 0.51%. It indicates that the appropriate amount of antioxidant 168 can effectively prevent the high-temperature thermo-oxidative degradation of PC products and reduce the degree of side reactions at high temperatures. When the dosage of antioxidant 168 is small, the antioxidant effect is not obvious and the colour of the obtained product is not good. After the dosage of antioxidant 168 exceeds 0.6 wt%, the yellow index of the product increases and the transparency decreases because the dosage of antioxidant 168 is too much, and its main component phosphite reacts with the weakly alkaline catalyst in a side reaction, which results in the weakening of the antioxidant effect of the antioxidant and the activity of the catalyst, and the appearance of the product has a bad colour.
The effect of antioxidant addition process on PC colour
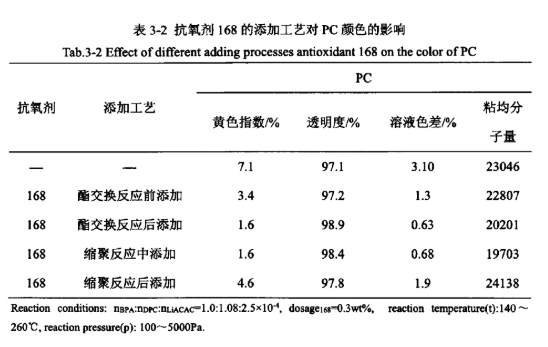
Due to the characteristics of the reaction of PC prepared by melt ester exchange method and the different properties of antioxidants, the different addition process of antioxidants may also have a certain effect on the appearance of PC colour. Table 3-2 examines the effects of different addition processes of antioxidant 168 on the colour of PC at the same dosage, respectively.
From Table 3-2, it can be seen that in the process of PC synthesis, several different addition processes of antioxidants have a greater impact on the appearance of the PC product colour, are different degrees of reduction of the yellow index of PC and the solution colour difference, improve its transparency, and basically have no effect on the viscosity average molecular weight of the product. The order of the effect of the addition process from excellent to poor is as follows: add after the ester exchange reaction ≥ add in the polycondensation reaction ≥ add before the ester exchange reaction > add after the polycondensation reaction. In addition, the effects of different addition processes of antioxidant BHT and antioxidant 2246 were investigated separately, and the results are shown in Table 3-3.
From Table 3-3 to see, antioxidant BHT and antioxidant 2246 add process effect of the order of excellence are: ester exchange reaction after adding > ester exchange reaction before adding, and Table 3-2 in the antioxidant 168 add process effect of excellence in the order of consistency, indicating that the antioxidant is mainly in the polycondensation reaction stage plays a role in the polymerisation phase, polycondensation stage when the reaction temperature is high, at this time to add the antioxidant can effectively prevent high temperature thermal degradation side reaction occurs. At this time, the addition of antioxidants can effectively prevent the occurrence of thermal degradation side reactions at high temperatures, and play a good antioxidant effect.
The effect of antioxidants on the performance of polycarbonate
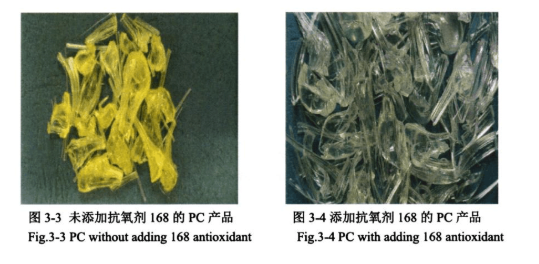
Through the experimental investigation of the above antioxidant, under the performance index of yellow index, transparency, solution colour difference and characteristic viscosity, it is concluded that the effect of antioxidant 168 is the best, Figures 3-3 and 3-4 are the appearance diagrams of PC products without the addition of this antioxidant and with the addition of this antioxidant, respectively.
Comparison of Figures 3-3 and 3-4 shows that the addition of antioxidant can significantly improve the appearance of PC product colour, but it is not known whether the addition of antioxidant will have a certain effect on the structural properties of PC, so the addition of 0.6 wt% of antioxidant 168 to the PC was carried out to characterize the product.
4.1 Infrared analysis
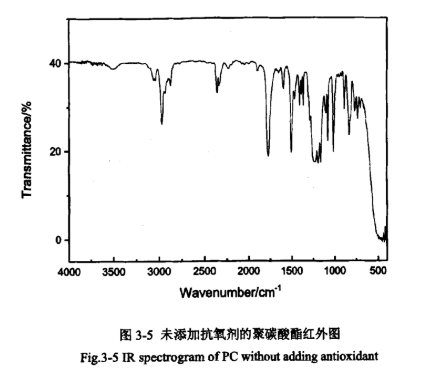
Infrared spectra can provide some information about chemical structure units, end groups, additives and crystalline state, etc. PC without antioxidant addition and PC with antioxidant addition were subjected to infrared characterisation, as shown in Figures 3-5 and 3-6.
From the infrared spectra of the samples in Figs. 3-5 and 3-6, it can be seen that the characteristic peaks of the two figures are basically the same. 1769cm-1 is the characteristic absorption peak of the stretching vibration of the planted group (C=O), which is located in the high-frequency side of the usual absorption of the carbonyl group due to the structure of the polycarbonate that makes the double bonding of its (C=O) enhancement, and therefore, the absorption is located in the high-frequency side of the usual absorption of the carbonyl group. 1219cm-1 and 1158cm-1 have a strong peak for the C-O stretching vibration peaks, so it can be determined that the sample contains an ester carbonyl group. 1503cm-1 has a medium intensity characteristic absorption peak, which is caused by the stretching vibration of the benzene ring skeleton, indicating that the sample contains a benzene ring. 2925cm-1, 2968cm-1, and 3042cm-1 are the characteristic absorption peaks of the stretching vibration of the C-H bond on the benzene ring. 1080cm-1, 1014cm-1, and 828cm-1 correspond to the characteristic absorption peaks of the stretching vibration of the C-H bond on the benzene ring, 828cm-1 correspond to the fingerprint peaks of the para-aromatic ring, which is basically consistent with the typical characteristic spectra of polycarbonate, and thus it can be determined that its main chain is a linear structure containing a polycarbonate group and a benzene ring, i.e., the sample is a linear bisphenol A-type polycarbonate. It also shows that the addition of antioxidants did not cause any change in the structure of PC.
4.2 Thermal stability
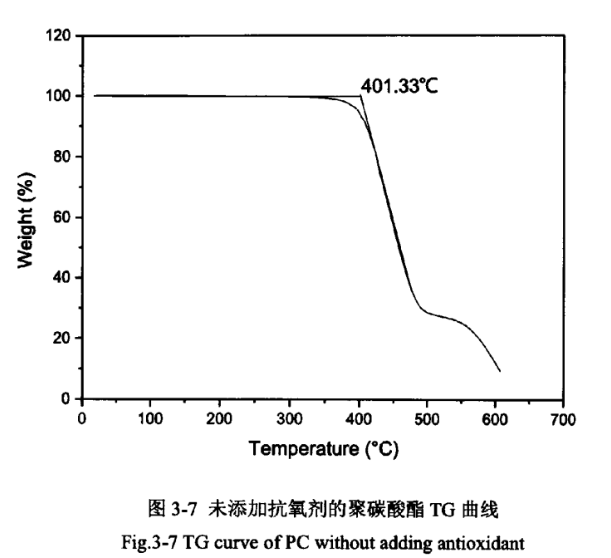
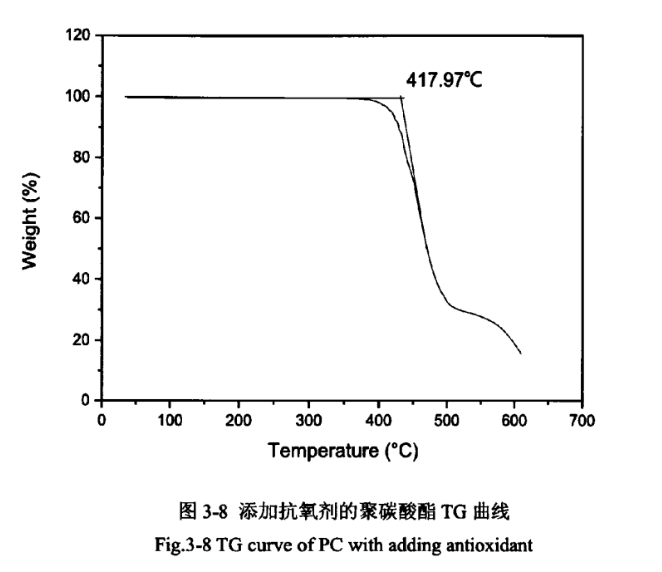
Due to the relatively high temperature of PC injection moulding, which is greater than 240℃, but PC starts to degrade in oxygen above 250℃. It has been reported in the literature that the thermal stability of PC can be effectively improved by adding antioxidants. The PC products without the addition of antioxidant and the addition of antioxidant were analysed thermogravimetrically, as shown in Figures 3-7 and 3-8.
As shown in Figures 3-7 and 3-8, the epitaxial onset temperature of PC products without added antioxidant was 401.33°C, while that of PC products with added antioxidant was 417.97°C. The thermal degradation temperature of PC was increased by 17°C, which indicates that the addition of antioxidant can significantly improve the thermal stability of PC products.
4.3 Differential scanning calorimetric analysis
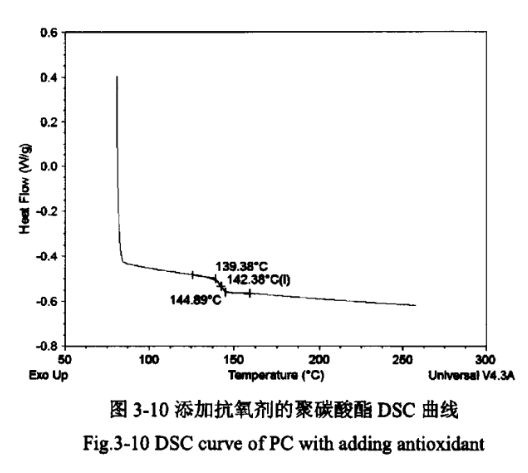
The glass transition temperature (Tg) is an important index for measuring resins, and usually resins used below the glass transition temperature are called hard plastics, and resins used above the glass transition temperature are called rubbers. Therefore, the glass transition temperature is a significant reference value for the later moulding and processing of polycarbonate coolers. Figures 3-9 and 3-10 show the DSC curves of PC products without added antioxidant and PC products with added antioxidant, respectively.
As can be seen from Figure 3-9 and Figure 3-10, the Tg of PC products in both cases is 142°C, which is similar to the glass transition temperature of PC standard products of 149°C, indicating that the addition of antioxidants to PC has basically no effect on its glass transition temperature. At the same time in the range of 230 ℃ ~ 270 ℃, the two curves are not found on the two curves of the obvious melting point turning point, indicating that the polycarbonate does not have a fixed melting point, that is, amorphous form.
Contact Us Now!
If you need Price, please fill in your contact information in the form below, we will usually contact you within 24 hours. You could also email me info@longchangchemical.com during working hours ( 8:30 am to 6:00 pm UTC+8 Mon.~Sat. ) or use the website live chat to get prompt reply.
| Formulation Products | ||
| CHLUMIAO® 1135 | CAS 125643-61-0 | Irganox 1135 / Antioxidant 1135 |
| CHLUMIAO® 1425 | CAS 65140-91-2 | Irganox 1425 / Dragonox 1425 / Antioxidant 1425 / BNX 1425 |
| CHLUMIAO® 1726 | CAS 110675-26-8 | Antioxidant 1726 / Irganox 1726 / Omnistab AN 1726 |
| CHLUMIAO® 3052 | CAS 61167-58-6 | IRGANOX 3052 / 4-methylphenyl Acrylate / Antioxidant 3052 |
| CHLUMIAO® 5057 | CAS 68411-46-1 | Irganox 5057 / Antioxidant 5057 / Omnistab AN 5057 |
| CHLUMIAO® 697 | CAS 70331-94-1 | Antioxidant 697 / Irganox 697 / Naugard XL-1 / Antioxidant 697 |
| CHLUMIAO® 80 | CAS 90498-90-1 | Irganox 80 / Antioxidant 80 |
| CHLUMIAO® 1024 | CAS 32687-78-8 | Irganox 1024 / Antioxidant 1024 |
| CHLUMIAO® 1035 | CAS 41484-35-9 | Irganox 1035 / Antioxidant 1035 |
| CHLUMIAO® HE-S01/N40 | ||
| CHLUMIAO® HN-55/70/80/502/510/514/516/602 | ||
| CHLUMIAO® HC-30/100 | ||
| CHLUMIAO® HO-17/17EH | ||
| CHLUMIAO® HS-502/503/504/603/605/608/101 | ||
| Phosphite antioxidants | ||
| CHLUMIAO® 168 | CAS 31570-04-4 | Irganox 168 / Antioxidant 168 |
| CHLUMIAO® 626 | CAS 26741-53-7 | Ultranox 626 / Irgafos 126 |
| CHLUMIAO® 1790 | CAS 40601-76-1 | Antioxidant 1790/ Cyanox 1790 / Irganox 1790 |
| CHLUMIAO® 245 | CAS 36443-68-2 | Irganox 245 / Antioxidant 245 |
| High Performance Phosphites | ||
| CHLUMIAO® 1500 | CAS 96152-48-6 | Antioxidant 1500 |
| CHLUMIAO® 4500 | CAS 13003-12-8 | Antioxidant 4500 |
| CHLUMIAO® PDP | CAS 80584-86-7 | PowerNox DHOP / Antioxidant DHOP |
| CHLUMIAO® 618 | CAS 3806-34-6 | Antioxidant 618 |
| CHLUMIAO® DLP | CAS 21302-09-0 | Antioxidant DLP |
| CHLUMIAO® DPP | CAS 4712-55-4 | Antioxidant DPP |
| CHLUMIAO® DTDP | CAS 36432-46-9 | Antioxidant DTDP |
| CHLUMIAO® THOP | CAS 80584-85-6 | Antioxidant THOP |
| CHLUMIAO® TNPP | CAS 26523-78-4 | Antioxidant TNPP / Tris(nonylphenyl) phosphite |
| CHLUMIAO® PEP-36 | CAS 80693-00-1 | Antioxidant 636 / Antioxidant 636 |
| CHLUMIAO® 9228 | CAS 154862-43-8 | Irganox 9228 / Antioxidant 9228 |
| CHLUMIAO® PEPQ | CAS 119345-01-6 | Hostanox PEPQ / Antioxidant PEPQ |
| Low Impurity Phosphites | ||
| CHLUMIAO® DPOP | CAS 15647-08-2 | 2-ethylhexyl diphenyl phosphite |
| CHLUMIAO® 8621 | CAS 68123-00-2 | Antioxidant 8621 |
| CHLUMIAO® DPDP | CAS 26544-23-0 | Antioxidant DPDP |
| CHLUMIAO® PDDP | CAS 25550-98-5 | Antioxidant PDDP |
| CHLUMIAO® PDOP | CAS 3164-60-1 | Antioxidant PDOP |
| CHLUMIAO® TPP | CAS 101-02-0 | Antioxidant TPP |
| CHLUMIAO® Poly(dicyclopentadiene-co-p-cresol) | CAS 68610-51-5 | Poly(dicyclopentadiene-co-p-cresol) |
| CHLUMIAO® SEED | CAS 42774-15-2 | Antioxidant SEED / Omnistab LS 5519 / Light Stabilizer 856 |
| Inhibited phenolic Antioxidants | ||
| CHLUMIAO® 264 | CAS 128-37-0 | Antioxidant 264 / Butylated hydroxytoluene |
| CHLUMIAO® 2,6-Di-tert-butylphenol | CAS 128-39-2 | 2,6-Di-tert-butylphenol |
| CHLUMIAO® 300 | CAS 96-69-5 | Irganox 300 / Antioxidant 300 |
| CHLUMIAO® 2246 | CAS 119-47-1 | Irganox 2246 / BNX 2246 |
| CHLUMIAO® 1222 | CAS 976-56-7 | Antioxidant 1222 / Irganox 1222 |
| CHLUMIAO® 702 | CAS 118-82-1 | Irganox 702 / Antioxidant 702 / Ethanox 702 |
| CHLUMIAO® DBHQ | CAS 88-58-4 | Antioxidant DTBHQ |
| CHLUMIAO® MTBHQ | CAS 1948-33-0 | 2-tert-butylhydroquinone Industrial Grade |
| CHLUMIAO® 1076 | CAS 2082-79-3 | Irganox 1076 / Antioxidant 1076 |
| CHLUMIAO® 1010 | CAS 6683-19-8 | Irganox 1010 / Antioxidant 1010 |
| CHLUMIAO® 1330 | CAS 1709-70-2 | Irganox 1330 / Ethanox 330 |
| CHLUMIAO® 1520 | CAS 110553-27-0 | Irganox 1520 / Antioxidant 1520 |
| Phenol Free Phosphites Antioxidants | ||
| CHLUMIAO® 8608 | CAS 26544-27-4 | Antioxidant AO DPD / Everaox 202 |
| CHLUMIAO® 430 | CAS 36788-39-3 | Antioxidant 430 / WESTON 430 |
| CHLUMIAO® 8608T | CAS 1334238-11-7, 69439-68-5 | Antioxidant 8608T |
| CHLUMIAO® 8627 | CAS 68610-62-8 | Antioxidant 8627 |
| CHLUMIAO® TDP | CAS 25448-25-3 | Antioxidant TDP |
| CHLUMIAO® TLP | CAS 3076-63-9 | Antioxidant TLP |
| CHLUMIAO® TOP | CAS 301-13-3 | Antioxidant TOP |
| CHLUMIAO® TTDP | CAS 77745-66-5 | Antioxidant TTDP |
| Thiol esters Antioxidants | ||
| CHLUMIAO® DLTDP | CAS 123-28-4 | Dilauryl thiodipropionate |
| CHLUMIAO® DSTDP | CAS 693-36-7 | istearyl thiodipropionate/ Antioxidant DSTDP |
| Aminic Antioxidants | ||
| CHLUMIAO® 3114 | CAS 27676-62-6 | Irganox 3114 / Antioxidant 3114 |
| CHLUMIAO® 4,4′-biphenol | CAS 92-88-6 | 4,4′-biphenol |
| Metal deactivators Antioxidants | ||
| CHLUMIAO® 1098 | CAS 23128-74-7 | Irganox 1098 / Antioxidant 1098 |